Recombinant human papillomavirus vaccine composition and use thereof
A technology of human papillomavirus and composition, which is applied in the field of recombinant human papillomavirus vaccine composition, can solve the problems of low vaccination completion rate, achieve the effects of reducing antigen immunization dose, high immune activity, and reducing the number of immunizations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of the vaccine composition containing HPV each type L1 VLP antigenic protein
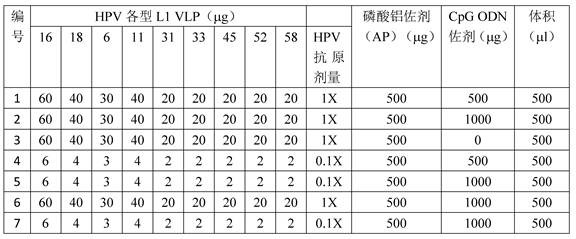
[0044] In order to study the technical effect of the vaccine preparation composition provided by the present invention. The inventors of the present invention have made the following various vaccine preparation compositions (0.5ml / dose), each preparation composition contains HPV various types of L1 VLP proteins (HPV16L1, 18L1, 6L1, 11L1, 31L1, 33L1, 45L1, 52L1, 58L1), aluminum adjuvant and CpG ODN adjuvant. The specific preparation method is as follows: Firstly, the stock solution of various types of HPV L1 VLP antigens is adsorbed on aluminum adjuvant (aluminum phosphate adjuvant AP, purchased from Zerun Biotechnology Co., Ltd.), and prepared into different ratios of various types of HPV L1 VLP antigens / aluminum Adjuvant (w / w) ratio of the adsorbed sample (here the aluminum adjuvant content is substantially the content of aluminum element); then add different c...
Embodiment 2
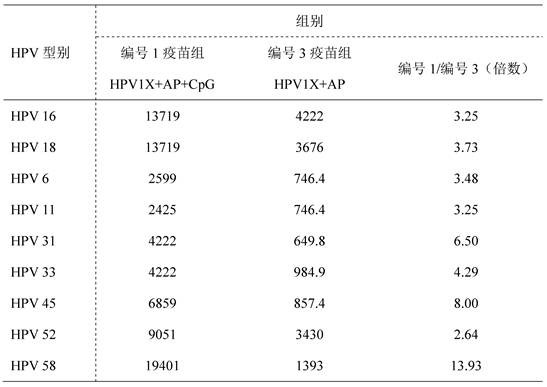
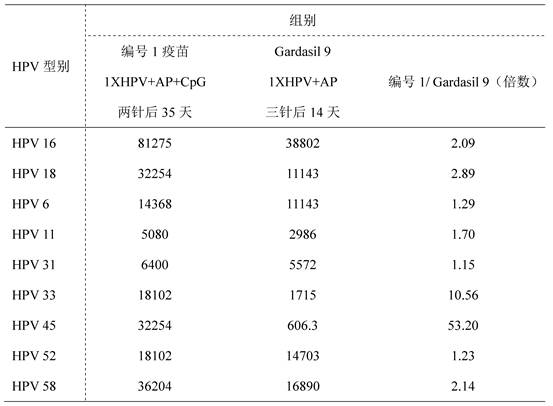
[0049] For obtaining the recombinant human papillomavirus vaccine preparation composition to be evaluated in Example 1. The inventors carried out immunogenicity studies using BALB / c mice as animal models. The immunogenicity of the vaccine preparation composition provided by the present invention was studied with various types of HPV L1 VLP proteins as antigens and aluminum adjuvant and CpG ODN as adjuvants. Select BALB / female mice aged 6-8 weeks and divide them into random groups, 10 mice in each group, intramuscularly inject vaccines prepared with HPV L1 VLP proteins of various types combined with adjuvant (Table 1), and the injection volume is 0.05ml (that is, 1 / 10 doses), set up a self-made vaccine group, a marketed vaccine Gardsail 9 group, and an adjuvant control group. One injection group was immunized on day 0, and blood was collected on day 35; Groups were immunized on days 0, 21, and 42, and blood was collected on day 56. Pseudovirion-Based Neutralization Assay (PB...
Embodiment 3
[0077] The inventors also studied the immunogenicity of new nine-valent HPV vaccines with different CpG ODN contents (such as 500~1000μg), and the immunogenicity of new nine-valent HPV vaccines containing different CpG ODN types (such as CpG7909 and CpG1018), and the results It shows that the new nine-valent HPV vaccines with various formulations have good immunogenicity. The mouse experiment procedure and neutralizing antibody detection method are the same as in Example 2, and the research results are shown in Table 6 below.
[0078] Table 6 GMT of mouse serum neutralizing antibody titers 14 days after two injections of each vaccine
[0079]
[0080] Note: See Table 1 for the formulations of No. 2, 4, 6 and 7 vaccines. The doses for mouse muscle immunization are all 1 / 10 doses, and the volume of immunization is 0.05ml.
[0081] It can be seen from Table 2-6 that the vaccine composition prepared according to the specific components and ratios provided by the present invent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com