A kind of trivalent genetic engineering subunit vaccine composition for preventing duck infectious serositis and preparation method thereof
An infectious and serositis technology, applied in the direction of genetic engineering, botany equipment and methods, biochemical equipment and methods, etc., can solve ducklings infected with infectious duck serositis, high safety requirements, immunogenic Poor sex and other problems, to achieve good immune effect, reduce production costs, small immune dose effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Construction of recombinant baculovirus
[0046] 1. Construction of transfer vector: truncation and multiple expression of antigenic proteins TbdR1, SIP, GldG, OmpA and HA of L. anatipestifer, the obtained protein fragments TbdR1-T3, SIP-T3, GldG-T3, OmpA -T3, HA-T3, CAMP and TrpB were entrusted by Anhui General Bio for gene synthesis, and connected to the commercial vector pFastBac I to obtain transfer vectors RA-A, RA-B, RA-C, RA-D, RA-E, RA-F and RA-G.
[0047] 2. Construction of recombinant baculovirus: The transfer vectors RA-A to RA-G synthesized in step 1 were respectively transferred into E. coli DH10 Bac competent cells, and positive clones were selected for PCR identification with M13 primer.
[0048] M13-F: TGTAAAACGACGGCCAGT
[0049] M13-R: CAGGAAACAGCTATGAC
[0050] The PCR reaction system was (total volume 25 μL): DNA template 0.5 μL, M13-F and M13-R 0.5 μL, DNA polymerase 12.5 μL and sterile water 11 μL.
[0051] PCR reaction conditions wer...
Embodiment 2
[0054] Example 2: Preparation of recombinant protein
[0055] 1. Recombinant baculovirus amplification: Inoculate the f1 generation recombinant baculoviruses rRA-A, rRA-B, rRA-C, rRA-D, rRA-E, rRA-F and rRA-G obtained in Example 1 respectively Insect cells sf9 were cultured at 27°C for 4 days, the culture was collected, and the supernatant was centrifuged to obtain the f2 generation recombinant baculovirus;
[0056] 2. Identification of expressed proteins:
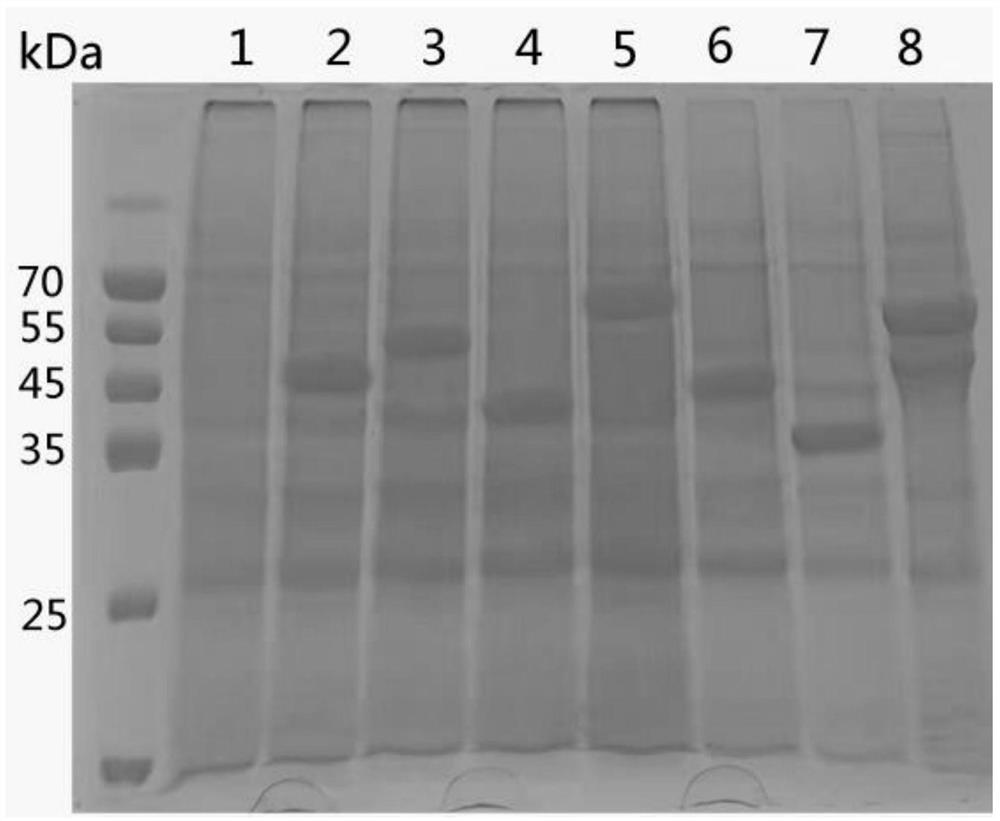
[0057] (1) The f2-generation recombinant baculoviruses rRA-A~rRA-G obtained in step 1 were respectively inserted into insect cells sf9 at an inoculum of MOI=5~10, cultured at 27°C for 4 days, the culture was collected, centrifuged to remove The supernatant obtained recombinant proteins TbdR1-T3, SIP-T3, CAMP, GldG-T3, OmpA-T3, TrpB and HA-T3;
[0058] (2) SDS-PAGE identification: The supernatant obtained in step 2 was subjected to SDS-PAGE electrophoresis; after electrophoresis, after staining and decolorization, it was ...
Embodiment 3
[0061] Example 3: Vaccine Preparation
[0062] 1. Inactivation: The TbdR1-T3, SIP-T3, CAMP, GldG-T3, OmpA-T3, TrpB and HA-T3 recombinant proteins prepared in Example 2 were added to the inactivation tank, and the final concentration was 0.2%. ~0.5% inactivator BEI, inactivated at 37°C for 24h.
[0063] 2. Semi-finished product inspection
[0064] (1) Sterility test: carry out sterility test according to the appendix of the current "Chinese Veterinary Pharmacopoeia".
[0065] (2) Determination of protein content: The protein content was detected by BCA method.
[0066] (3) Inactivation test: The inactivated recombinant proteins were respectively inserted into insect cells sf9, and cultured at 27°C for 72 hours. No lesions were observed, and the inactivation test was determined to be qualified.
[0067] 3. Preparation of vaccine vaccine composition:
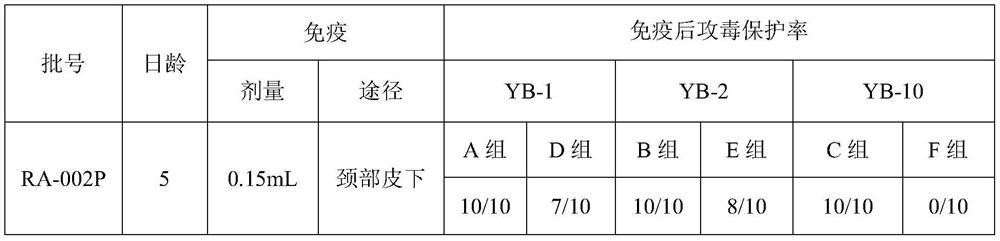
[0068] The semi-finished protein antigen after passing the inspection is used for vaccine preparation (each liquid component...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com