Combined live vaccine against porcine reproductive and respiratory syndrome and swine fever, and application thereof
A technology for respiratory syndrome and swine fever vaccine, which is applied in the field of preparation of porcine reproductive and respiratory syndrome and swine fever combined live vaccine, can solve the problem of time-consuming, increased cost, multiple injections, multiple doses, and difficulty in multiple immunization procedures, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
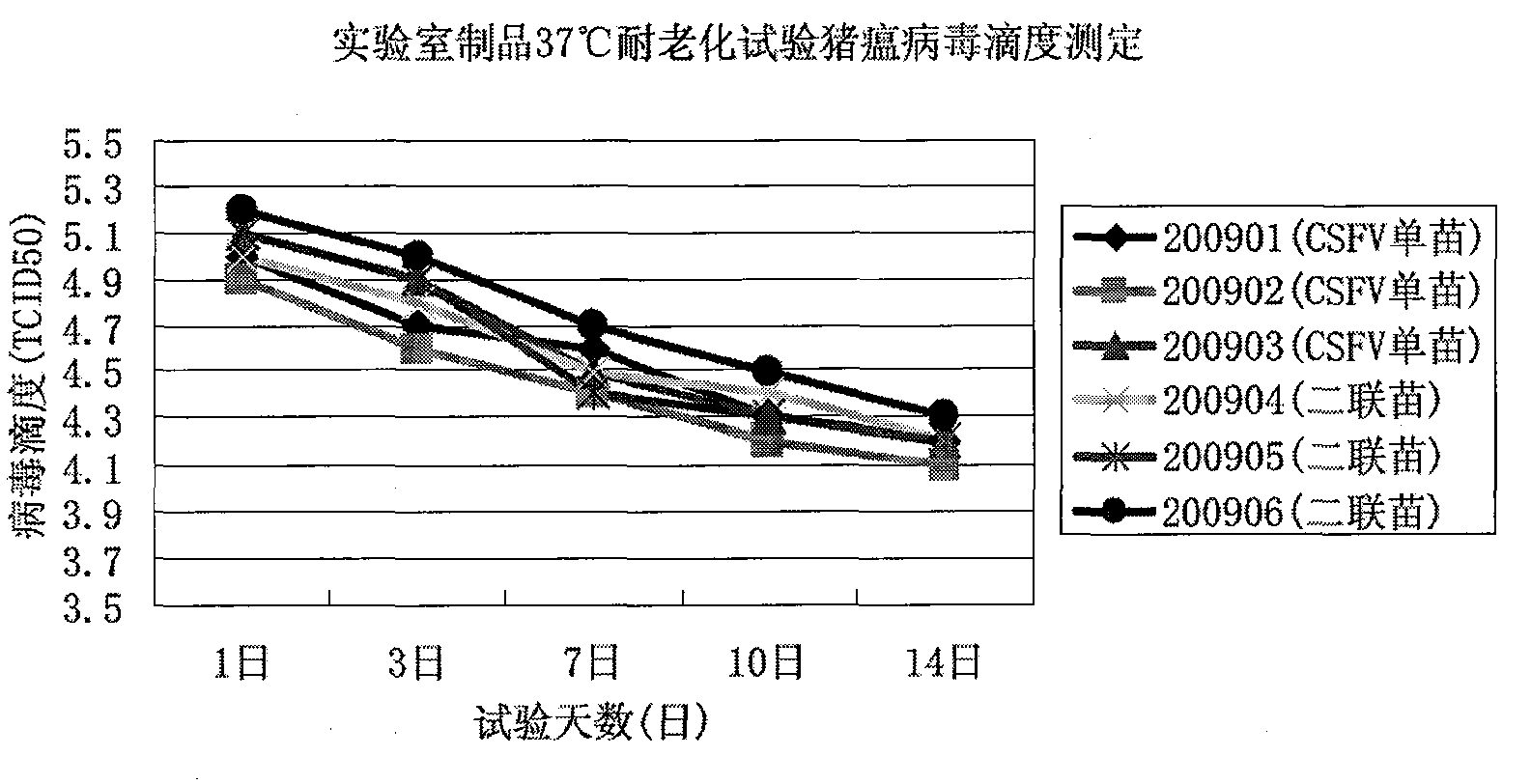
Image
Examples
Embodiment 1
[0050] (1) Passage and cultivation of cells for seedling preparation: the Marc-145 cell line used for the production of highly pathogenic porcine reproductive and respiratory syndrome TJM strain virus was digested and passed on by trypsin-EDTA cell dispersion, and passed on one to three; The BT cell line used for the production of the attenuated strain of hog fever lathe virus was digested and passaged with trypsin-EDTA cell dispersion, and the passage was carried out at a ratio of 1 to 5; the cell growth solution was added to continue culturing at 37°C, and when a good monolayer was formed, it was used for further passage. passaging or inoculation of viruses;
[0051](2) Propagation of cytotoxic species: the highly pathogenic porcine reproductive and respiratory syndrome TJM strain virus species is inoculated according to the inoculation amount of MOI0.01-0.5, cultivated for 3-5 days after inoculation, when the cytopathic effect reaches 70% Harvest the virus liquid; make a 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com