Optimized PCV2d ORF2 gene and preparation method of virus-like particles
A porcine circovirus and plasmid technology, applied in the field of molecular biology, can solve the problems of poor Cap protein solubility, low Cap protein yield, and low VLPs formation rate, and achieve the effect of shortening the experimental period, mature technology and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
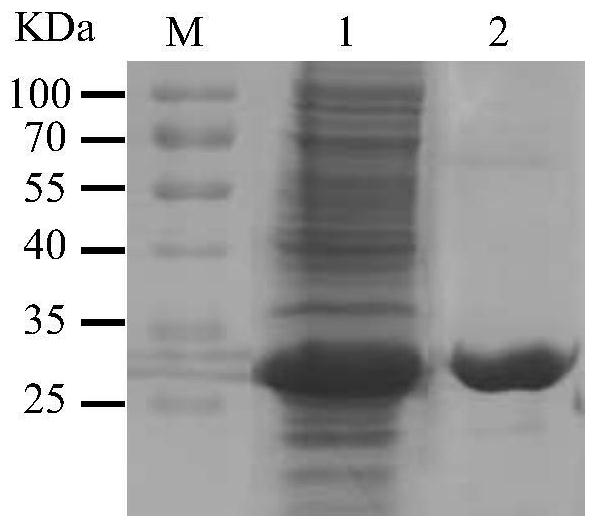
Image
Examples
Embodiment 1
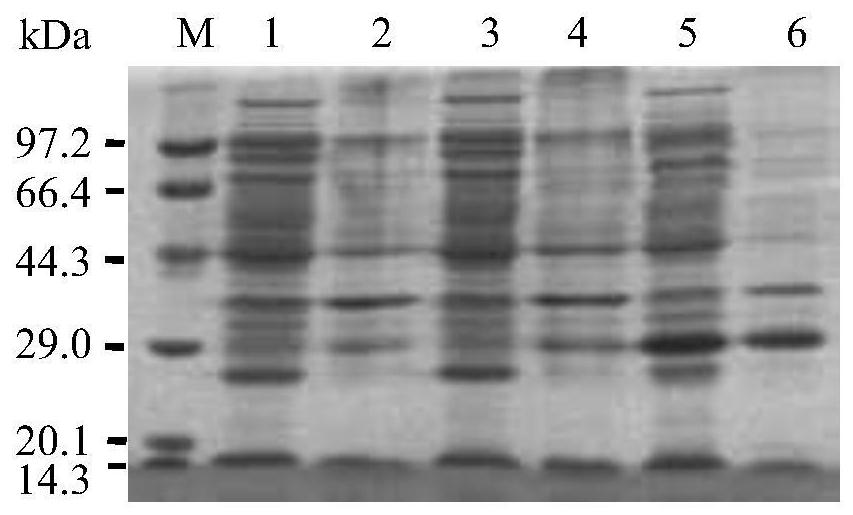
[0069] According to the ORF2 gene in the genome of PCV2d HB-MC1 strain, and optimized according to the codon preference of Escherichia coli, an NdeI restriction site was introduced at its 5' end, a stop codon and a Hind III restriction site were introduced at the 3' end, After the gene is synthesized, it is cloned into the pET30a(+) expression vector by double enzyme digestion to obtain the recombinant expression plasmid carrying the target fragment. The CAI (Codon Adaptation Index) of the original sequence was only 0.63, which was increased to 0.85 after optimization, which greatly increased the expression level and the probability of soluble expression.
Embodiment 2
[0071] The positive recombinant expression plasmid pET30a-ORF2 was transformed into E.coli Rosetta (DE3) host expression bacteria, positive colonies were picked on the LB plate containing kanamycin resistance for sequencing and identification, and the sequences without mutations and frameshifts were obtained. Positive recombinant expression strain Rosetta-pET30a-ORF2.
Embodiment 3
[0073] Pick a single positive recombinant colony, shake and expand in LB medium containing 20 μg / mL kanamycin at 37°C at a shaking speed of 200 r / min for 12 hours, then mix the overnight culture with a ratio of 1:100 Re-inoculate in fresh LB medium containing 20 μg / mL kanamycin, shake at 200 r / min at 37°C until OD 600 After about 0.7, different induction conditions were used to induce the expression of the target protein.
[0074] Four induction temperatures of 27°C, 32°C, 37°C and 42°C were used to induce the expression of the engineered bacteria for the same time, and the maximum amount of soluble target protein was obtained at the induction temperature of 37°C.
[0075] 1. Under the induction temperature of 37°C, the engineered bacteria were induced and expressed for 1h, 2h, 3h, 4h, 5h, 6h, 7h and 8h, and the maximum amount of soluble target protein was obtained after the induction time of 6h.
[0076] 2. At an induction temperature of 37°C, add IPTG with a final concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com