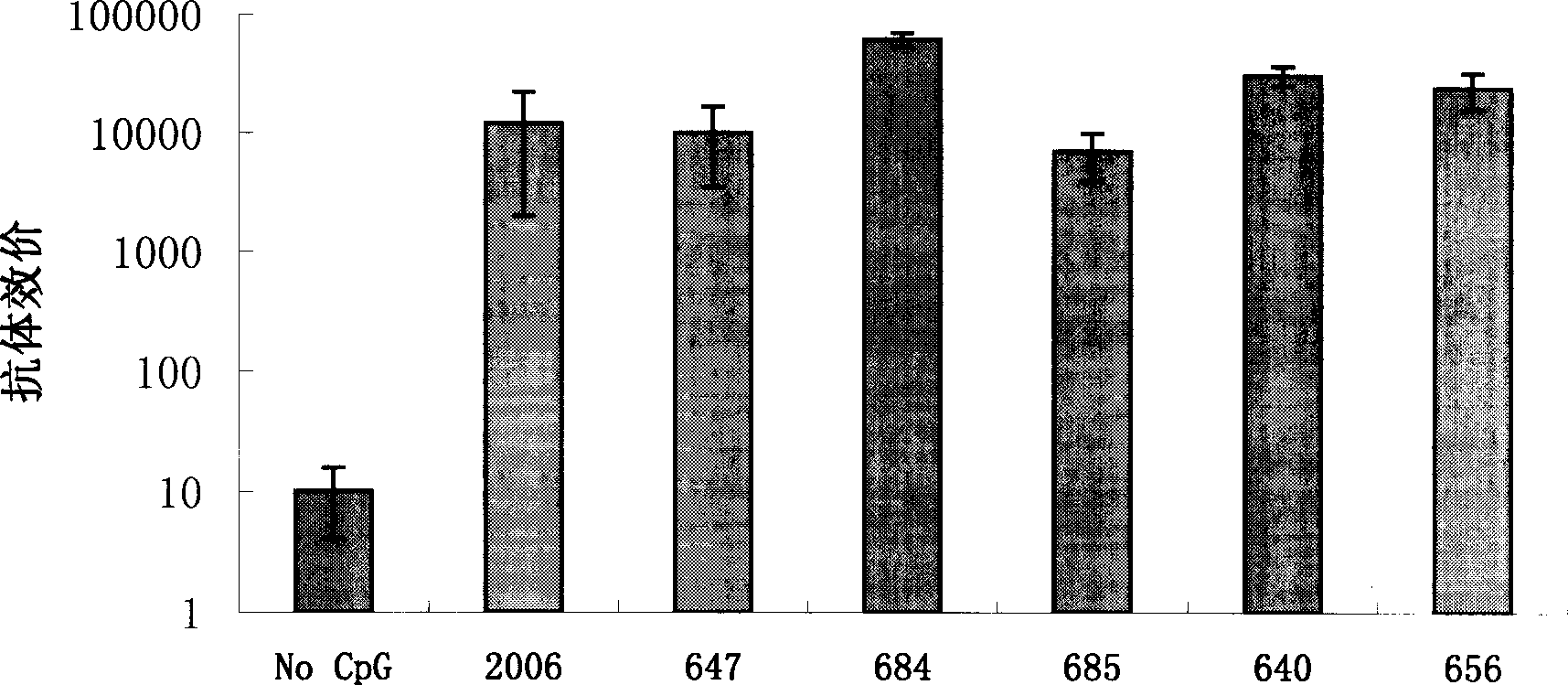
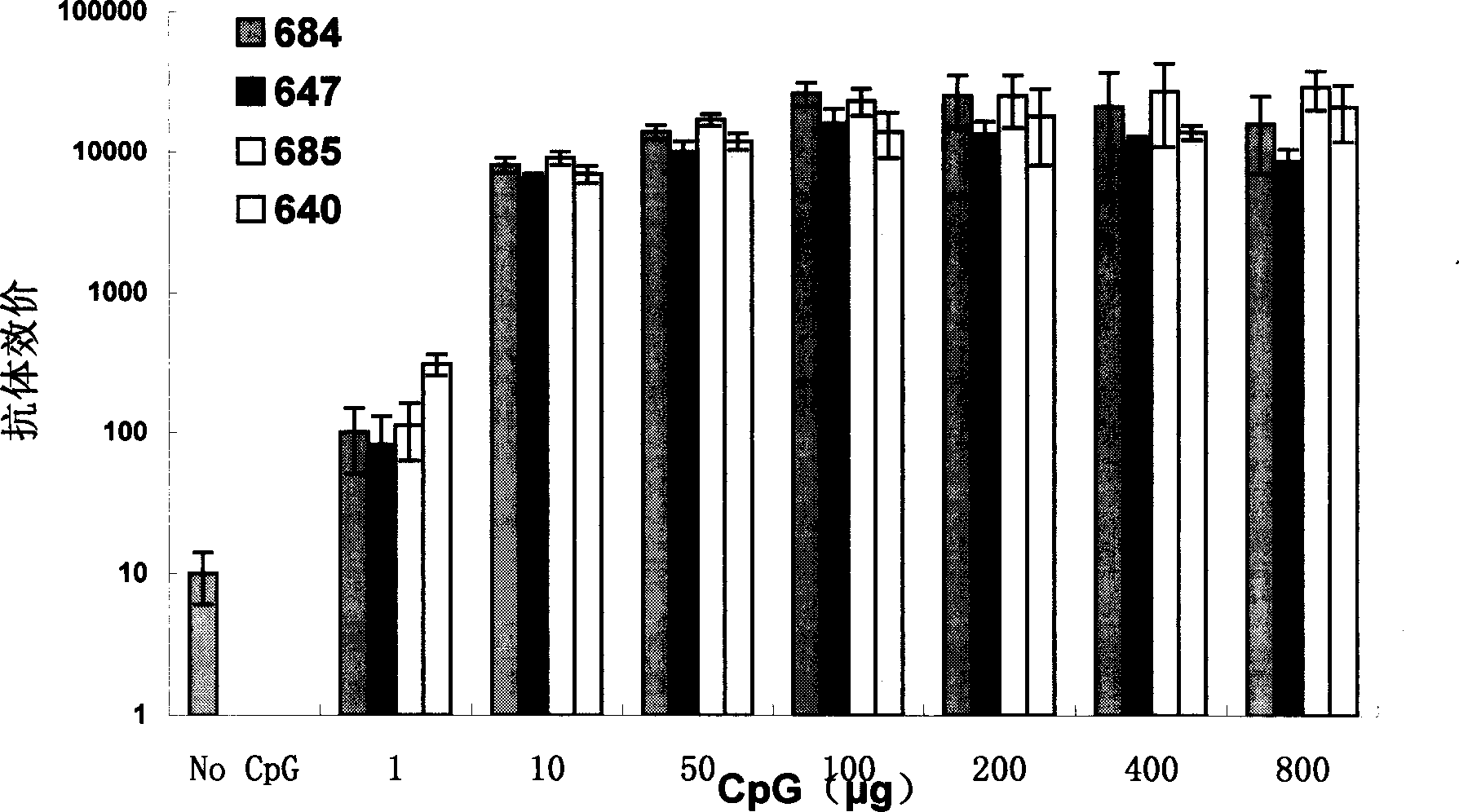
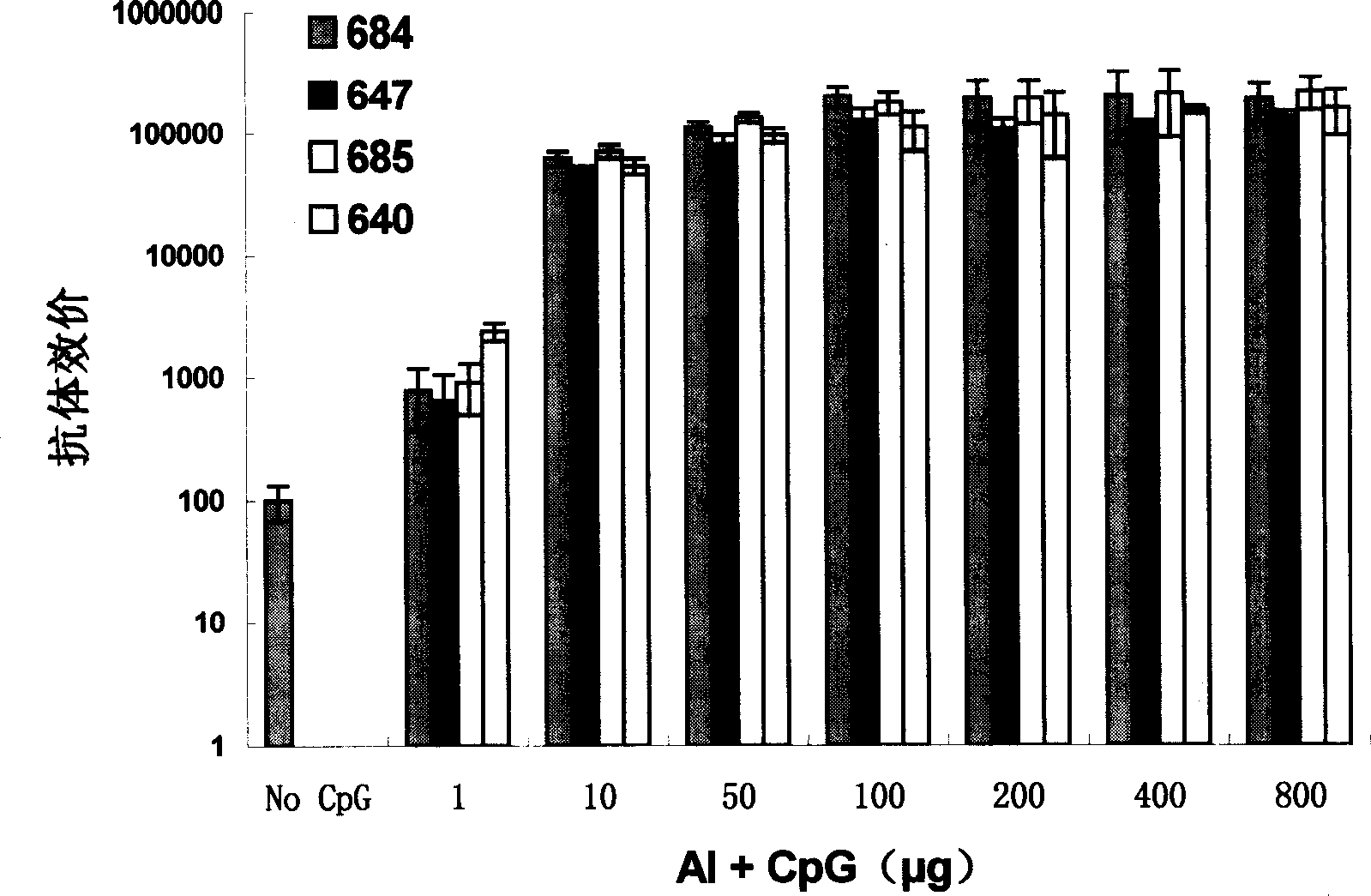
Deoxy nucleotide containing CpG single chain as adjuvant of hepatitis B virus vaccine
A hepatitis B virus, deoxynucleotide technology, applied in the direction of antiviral agents, medical preparations containing active ingredients, sugar derivatives, etc. and other problems to achieve the effect of prolonging the time limit of immune response, reducing the number of immunizations, and enhancing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The design of embodiment 1CpG single-stranded deoxynucleotide
[0036] The design sequence is as follows:
[0037] (G)n(L)nX1X2CGY1Y2(M)n(G)n
[0038] X1=A, T, G; X2=A, T; Y1=A, T; Y2=A, T, C; L, M=A, T, C, G; n is 0-6
[0039] 5'-ggggTCgTTCgTCgTTgggggg-3' (SEQ ID NO: 1) [121]
[0040] 5'-ggggATAACgTTgCgggggg-3' (SEQ ID NO: 2) [143]
[0041] 5'-ggggTgCAACgTTCAgggggg-3' (SEQ ID NO: 3) [402]
[0042] 5'-ggggTCCTACgTAggAgggggg-3' (SEQ ID NO: 4) [123]
[0043] 5'-ggggTCCATgACgTTCCTgAAgggggg-3' (SEQ ID NO: 5) [603]
[0044] 5'-gggggACgTCgCCgggggggg-3' (SEQ ID NO: 6) [118]
[0045] 5'-ggATCCgTACgCATgggggg-3' (SEQ ID NO: 7) [320]
[0046] 5'-ggggggAATCgATTCgggggg-3' (SEQ ID NO: 8) [154]
[0047] 5'-gggATgCATCgATgCATCgggggg-3' (SEQ ID NO: 9) [464]
[0048] 5'-ggTgCgACgTCgCAgggggg-3' (SEQ ID NO: 10) [471]
[0049] 5'-gggACgTACgTCgggggg-3' (SEQ ID NO: 11) [390]
[0050] 5'-gggggATCgACgTCgATCgggggg-3' (SEQ ID NO: 12) [322]
[0051] 5'-ggCgATCgATCgATCggggggg-3' (SEQ ID ...
Embodiment 2
[0232] Synthesis of embodiment 2CpG single-stranded deoxynucleotides
[0233] The solid-phase phosphoramidite triester method is used to synthesize DNA fragments. This method has the advantages of high efficiency and rapidity, and has been widely used in DNA chemical synthesis.
[0234] DNA chemical synthesis is different from enzymatic DNA synthesis. The enzymatic DNA synthesis process extends from the 5'→3' direction, while DNA chemical synthesis starts from the 3' end. Concrete reaction steps are as follows:
[0235] 1. Deprotection group
[0236] Use trichloroacetic acid to remove the protective group DMT (dimethoxytrityl) of the nucleotide attached to CPG (Controlled Pore Glass) to obtain a free 5'-hydroxyl terminal for the next condensation reaction use.
[0237] 2. Activation
[0238] The phosphoramidite-protected nucleotide monomer is mixed with tetrazolium activator and enters the synthesis column to form phosphoramidite tetrazolium active intermediate (its 3'-end...
Embodiment 3
[0248] Embodiment 3 detects hepatitis B virus antibody with ELISA method
[0249] 1. Reagents
[0250] 1. Preparation of HBsAg (without aluminum adjuvant, Beijing Institute of Biological Products) vaccine: 1 mg of HBsAg protein lyophilized powder was dissolved in 1 ml of PBS to prepare an application solution (1 mg / ml).
[0251] 2. HRP-horse anti-mouse secondary antibody: Beijing Dingguo Biotechnology Co., Ltd.
[0252] 3. PBS: 1000ml
[0253] NaCl………………………………… 8g (Beijing Chemical Plant)
[0254] KCl………………………………… 0.2g (Beijing Chemical Plant)
[0255] Na 2 HPO 4 12H 2 O………………………… 2.9g (Beijing Chemical Plant)
[0256] K H 2 PO4…………………………………… 0.2g (Beijing Chemical Plant)
[0257] After fully dissolving with 800ml of ultrapure water, adjust the pH to 7.2-7.4 with HCl or NaOH, and set the volume to 1000ml
[0258] 4. Coating solution: 100ml
[0259] PBS…………………………………80ml
[0260] 50% Glutaraldehyde…………………… 1.6ml (Beijing Chemical Plant)
[0261] After fully dissolved,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com