Combined Newcastle disease and infectious bronchitis inactivated vaccine and method for preparing same
A technology of inactivated vaccines and Newcastle disease, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as difficulties, achieve simple and fast operation, save man-hours, and benefit absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] New, branch dual inactivated vaccine prepared by the present invention of embodiment 1
[0025] The LaSota strain of Newcastle disease virus is used to manufacture this product, and the virulent Beijing strain of chicken Newcastle disease virus (CVCC AV1611) is preferably used for efficacy testing. The infectious bronchitis virus strain used for seedling production and effect test is M41 strain.
[0026] Dilute the Newcastle disease virus seed LaSota strain virus seed into 10 4 EID 50 , the infectious bronchitis strain M41 was diluted to 10 6 EID 50 , after dilution, mix the two virus solutions at a ratio of 1:1. Inoculate 9-11-day-old susceptible chicken embryos with mixed virus seeds, inoculate 0.1ml of allantoic cavity in each embryo, seal the inoculation hole and stomata with wax, incubate at 37°C, discard dead embryos before 72 hours, and collect dead embryos 72-96 hours And 96h living embryo embryo fluid.
[0027] The embryo fluid was inactivated by 0.1% for...
Embodiment 2
[0028] The new and branch dual inactivated vaccine containing aluminum stearate produced by the conventional heteroembryon inoculation of embodiment 2
[0029] The LaSota strain of Newcastle disease virus is used to manufacture this product, and the virulent Beijing strain of chicken Newcastle disease virus (CVCC AV1611) is preferably used for efficacy testing. The infectious bronchitis virus strain used for seedling production and effect test is M41 strain.
[0030] 2. Inoculate 9-11-day-old susceptible chicken embryos with Newcastle disease virus species LaSota strain virus seeds and infectious bronchitis virus strain M41 respectively, and each embryo is inoculated with 0.1ml of the allantoic cavity, and the inoculation hole and stomata are sealed with wax. Incubate at 37°C, discard dead embryos before 72 hours, collect dead embryos at 72-96 hours and live embryos at 96 hours.
[0031]3. The harvested embryo fluid was inactivated by 0.1% formaldehyde at 37°C for 20 hours, a...
Embodiment 3
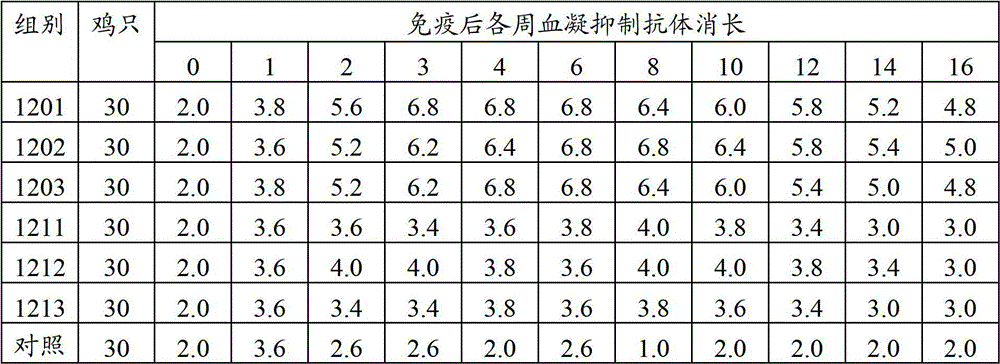
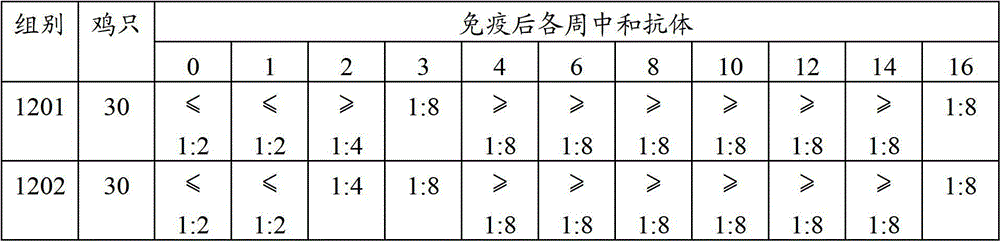
[0032] The new, branch dual inactivated vaccine character comparison that embodiment 3 two kinds of methods produce
[0033] 1. Appearance: Both are milky white homogeneous emulsions.
[0034] 2. Dosage form: Both are water-in-oil type. Drop a drop of oil emulsion on clean water, observe its shape, whether it disperses, and the time it disperses. The results showed that both were regular circular in shape and non-diffusion.
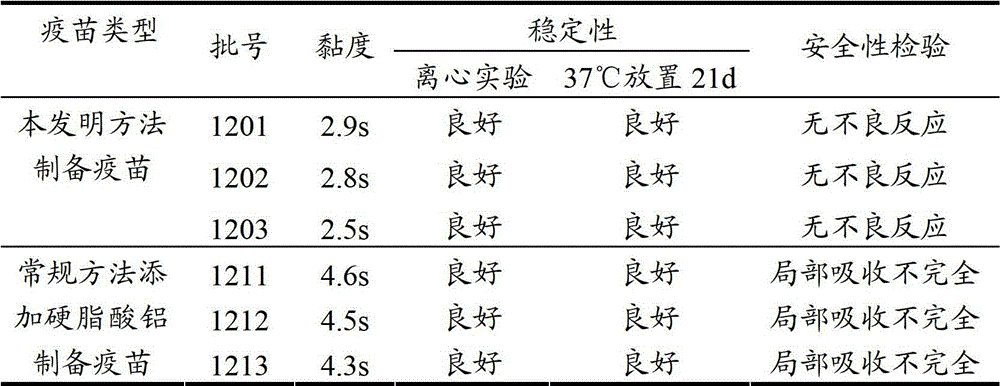
[0035] 3. Viscosity: Use a 1ml straw (inner diameter of the upper mouth is 2.7mm, and inner diameter of the lower mouth is 1.2mm), absorb 1ml of vaccine at about 25°C, let it flow out vertically and naturally, and record the time required for the outflow of 0.4ml. The results showed that the three outflow times of the two were all within 5s, which met the requirements of the regulations, and the viscosity of the vaccine prepared by the method of the present invention was lower, all within 3s.
[0036] 4. Stability:
[0037] (1) Take 10ml of the vaccin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com