Adjuvant for vaccine, vaccine composition containing adjuvant and application of vaccine composition
A vaccine composition and adjuvant technology, which is applied in the direction of drug combination, medical preparations containing active ingredients, bacterial antigen components, etc., can solve the needs of the development of new vaccines, the variability of aluminum glue vaccines, and the impact on the nervous system To achieve the effect of long-lasting antibody, stable effect and less frequency of immunization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
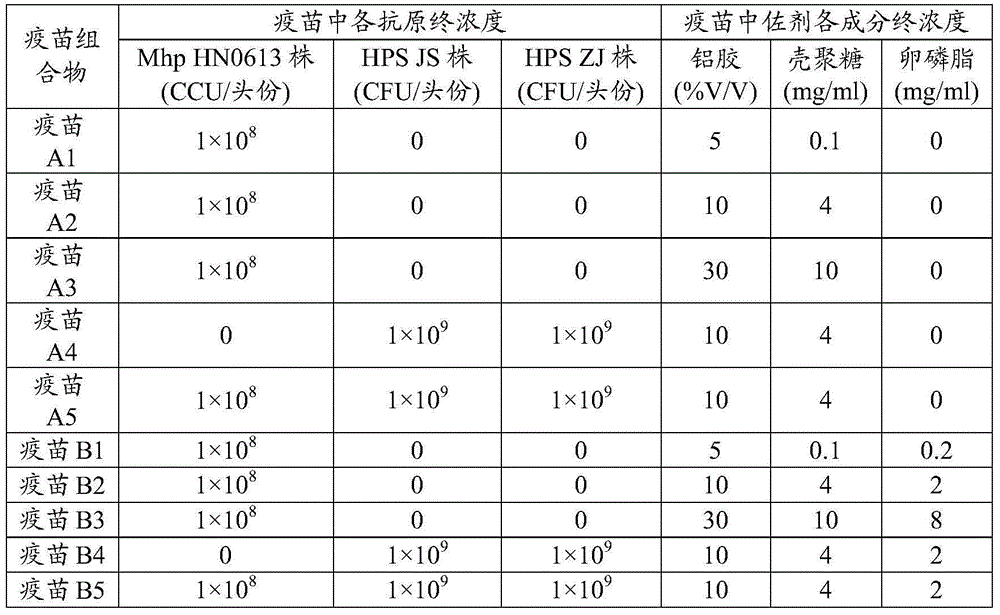
[0034] As an embodiment of the present invention, in the vaccine composition of the present invention, the aluminum gel content is 8%-20% V / V, and the chitosan concentration is 1-5 mg / ml.
[0035] As a preferred embodiment of the present invention, in the vaccine composition of the present invention, the aluminum gel is 8%-20% V / V, the chitosan is 1-5mg / ml, and the lecithin concentration is 0.5-5mg / ml. ml.
[0036] As an embodiment of the present invention, in the vaccine composition of the present invention, the antigens include Mycoplasma hyopneumoniae antigen, Haemophilus parasuis antigen, Pasteurella multocida antigen, Streptococcus suis antigen, grapevine Bacillus antigen, Bordetella bronchiseptica antigen, Actinobacillus lobar pneumonia antigen, Escherichia coli antigen, Bordetella bronchiseptica antigen and toxigenic Pasteurella multocida antigen, Salmonella choleraesuis antigen, Salmonella enteritidis antigen, One or more of Erysipelothrix rhusiopathiae antigen, Mycop...
Embodiment 1
[0054] The preparation of embodiment 1 antigen
[0055] 1.1 Preparation of Mycoplasma hyopneumoniae antigen
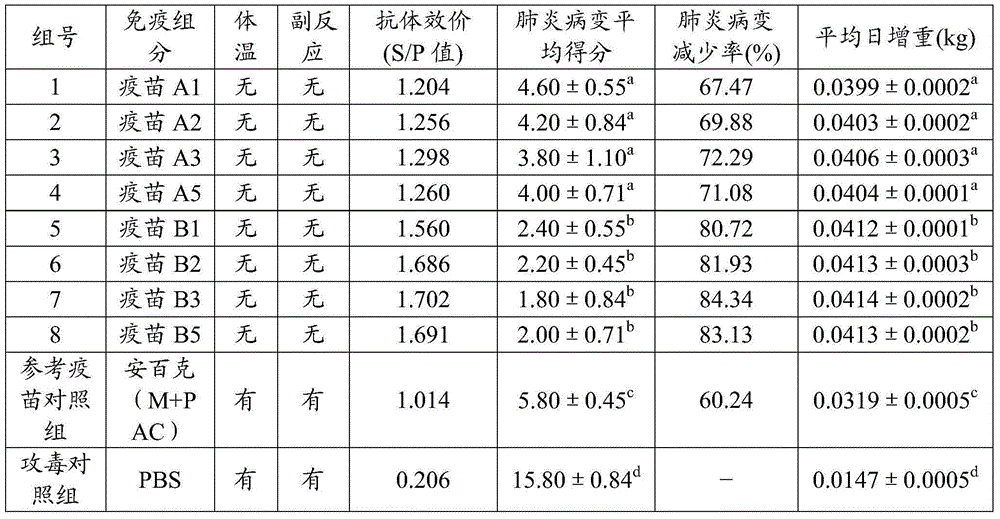
[0056] According to the Chinese patent CN103083655A, the bacterial liquid of Mycoplasma hyopneumoniae HN0613 strain was prepared and the content of bacterial liquid was determined, and the result: the content of Mycoplasma hyopneumoniae strain HN0613 was 1×10 9 CCU / ml; and inactivate and test the bacterial liquid, the result: the coloring of the medium does not change, and the pH value of the medium does not decrease, indicating that the bacterial liquid of Mycoplasma hyopneumoniae HN0613 strain is successfully inactivated.
[0057] 1.2 Preparation of Haemophilus parasuis antigen
[0058] According to Chinese patent CN103083655A, respectively prepare Haemophilus parasuis type 4 JS strain and type 5 ZJ strain bacterial liquid and measure the bacterial liquid content, the result: the contents of Haemophilus parasuis type 4 JS strain and type 5 ZJ strain are both 5× 10 ...
Embodiment 2
[0059] The preparation of embodiment 2 adjuvants
[0060] Aluminum glue: purchased from General Chemical, LLC of the United States HPA adjuvant.
[0061] Chitosan mother liquor: the chitosan purchased from Qingdao Haihui Biological Engineering Co., Ltd. (molecular weight is 145kD, and the deacetylation degree is 50%) is completely dissolved in 1% V / V acetic acid aqueous solution and is prepared with a concentration of 15mg / ml Chitosan acetic acid solution, adjust the solution to pH 7.4 with NaOH, and carry out filter sterilization to obtain 15mg / ml chitosan mother liquor.
[0062] Lecithin mother liquor: Dissolve 1 g of lecithin purchased from Beijing Yuanhuamei Phospholipid Technology Co., Ltd. with ethanol, add PBS solution and mix evenly, then conduct vacuum distillation. Steam sterilize at -125°C for 30 minutes, stir twice with ultrasonic waves, 10 minutes each time, add PBS solution to 100ml, and perform filter sterilization to obtain 10mg / ml lecithin mother liquor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com