Adjuvant used for vaccine and application thereof
An adjuvant and vaccine technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve potential safety hazards, can not meet the needs of new vaccine development, toxic and side effects and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 antigen
[0051] 1.1 Preparation of porcine circovirus antigen
[0052] According to Chinese patent CN103083655A, porcine circovirus type 2 SH strain virus liquid was prepared and the virus liquid content was measured, and the result: the content of porcine circovirus type 2 SH strain was 5×10 6.0 TCID 50 / ml; and inactivate the virus liquid and detect the inactivation effect with the indirect immunofluorescence method IFA, the result: no green PCV2 positive cells are produced, indicating that the porcine circovirus type 2 SH strain virus liquid is successfully inactivated.
[0053] 1.2 Preparation of Mycoplasma hyopneumoniae antigen
[0054] According to the Chinese patent CN103083655A, the bacterial liquid of Mycoplasma hyopneumoniae HN0613 strain was prepared and the content of bacterial liquid was determined, and the result: the content of Mycoplasma hyopneumoniae strain HN0613 was 1×10 9 CCU / ml; and inactivate and test the bacteri...
Embodiment 2
[0057] The preparation of embodiment 2 adjuvants
[0058] Aluminum glue: purchased from General Chemical, LLC of the United States HPA adjuvant.
[0059] Levamisole mother solution: The levamisole raw material purchased from Xi'an Saino Biotechnology Co., Ltd. was heated and dissolved into a 40 mg / ml solution, and sterilized by filtration to obtain a 40 mg / ml levamisole mother solution.
[0060] Sodium polyacrylate mother liquor: mix 1g sodium polyacrylate purchased from Shanghai Yiji Industrial Co., Ltd. with 100% distilled water, heat and boil until completely dissolved, then cool to room temperature, that is, configure a sodium polyacrylate mother liquor with a concentration of 10mg / ml, and Autoclave at 121°C for 30 minutes before use.
Embodiment 3
[0061] Preparation and animal test of embodiment 3 inactivated vaccine composition
[0062] 3.1 Preparation of inactivated vaccine composition
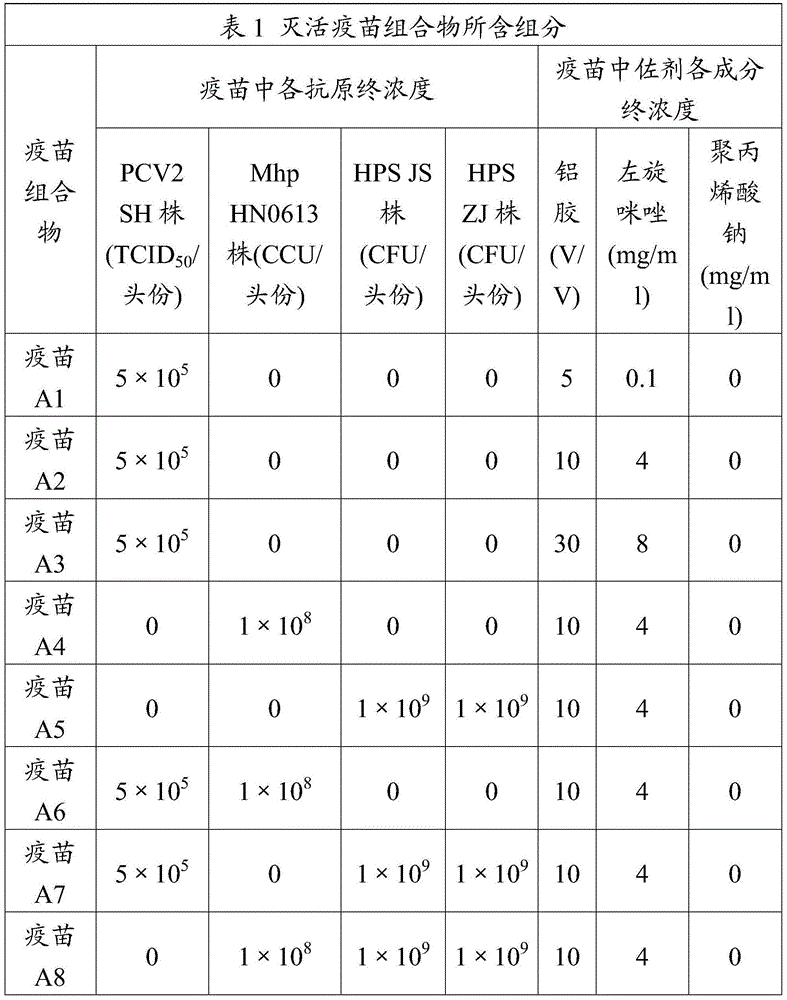
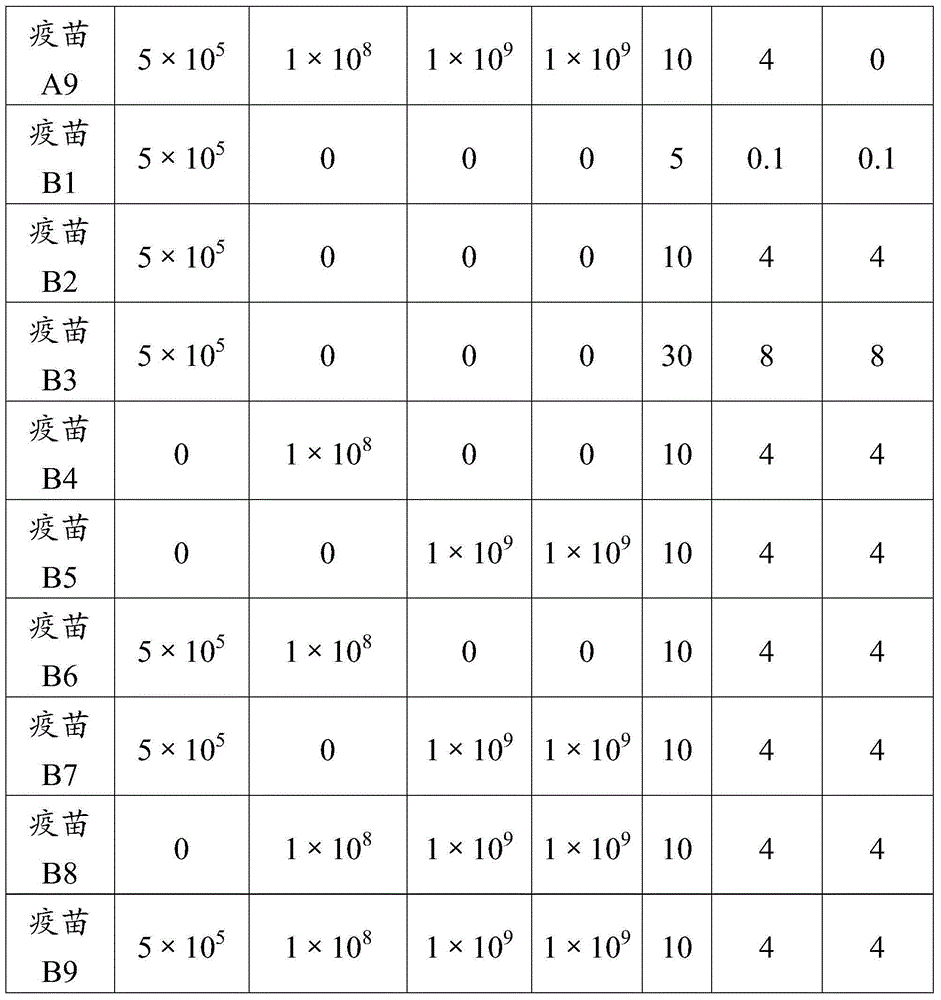
[0063] The antigen prepared in Example 1 and the adjuvant prepared in Example 2 were diluted with PBS solution, and each antigen solution after dilution was mixed with the final concentration of the adjuvant according to the components contained in the inactivated vaccine composition in Table 1, with 500 Stir at -800 rpm for 10-15 minutes, add 1% V / V of thimerosal (purchased from Sinopharm Chemical Reagent Co., Ltd.) solution before terminating the stirring, so that the final concentration does not exceed 1 / 10,000, fully shake Mix well, subpackage, and store at 2-8°C after subpackaging.
[0064]
[0065]
[0066] 3.2 Animal Test of Porcine Circovirus Antigen of Inactivated Vaccine Composition
[0067] Vaccines A1, A2, A3, A6, A7, A9, B1, B2, B3, B6, B7, B9 prepared in Example 3.1 and a reference vaccine control group (PCV2 vac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com