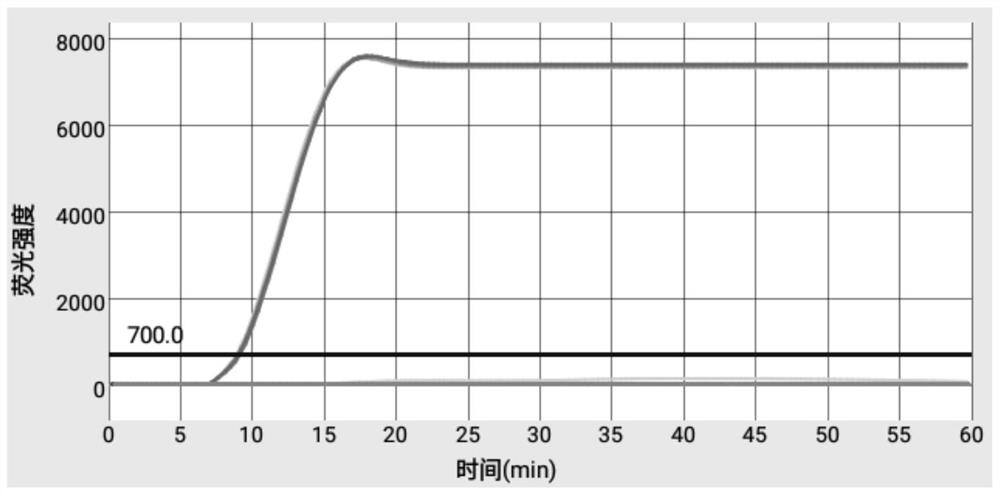
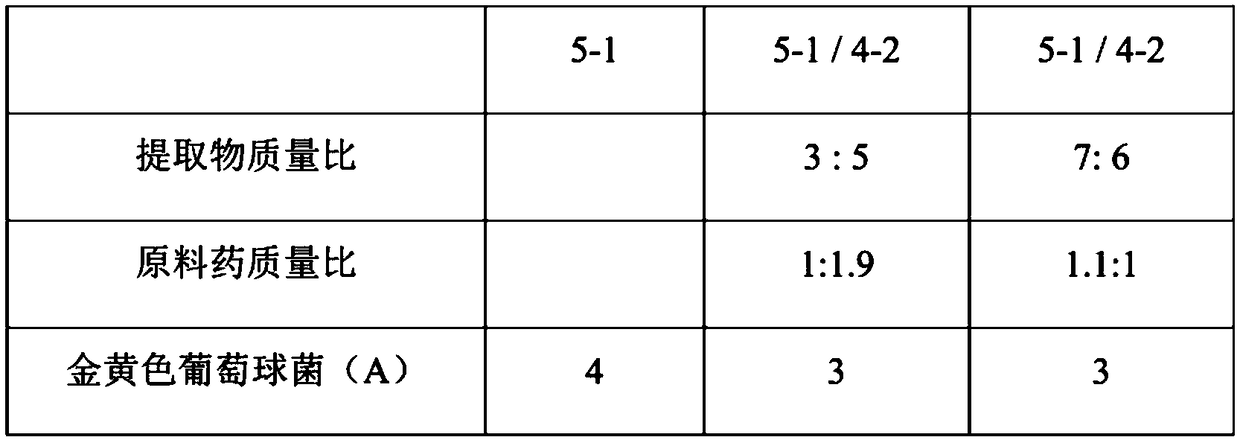
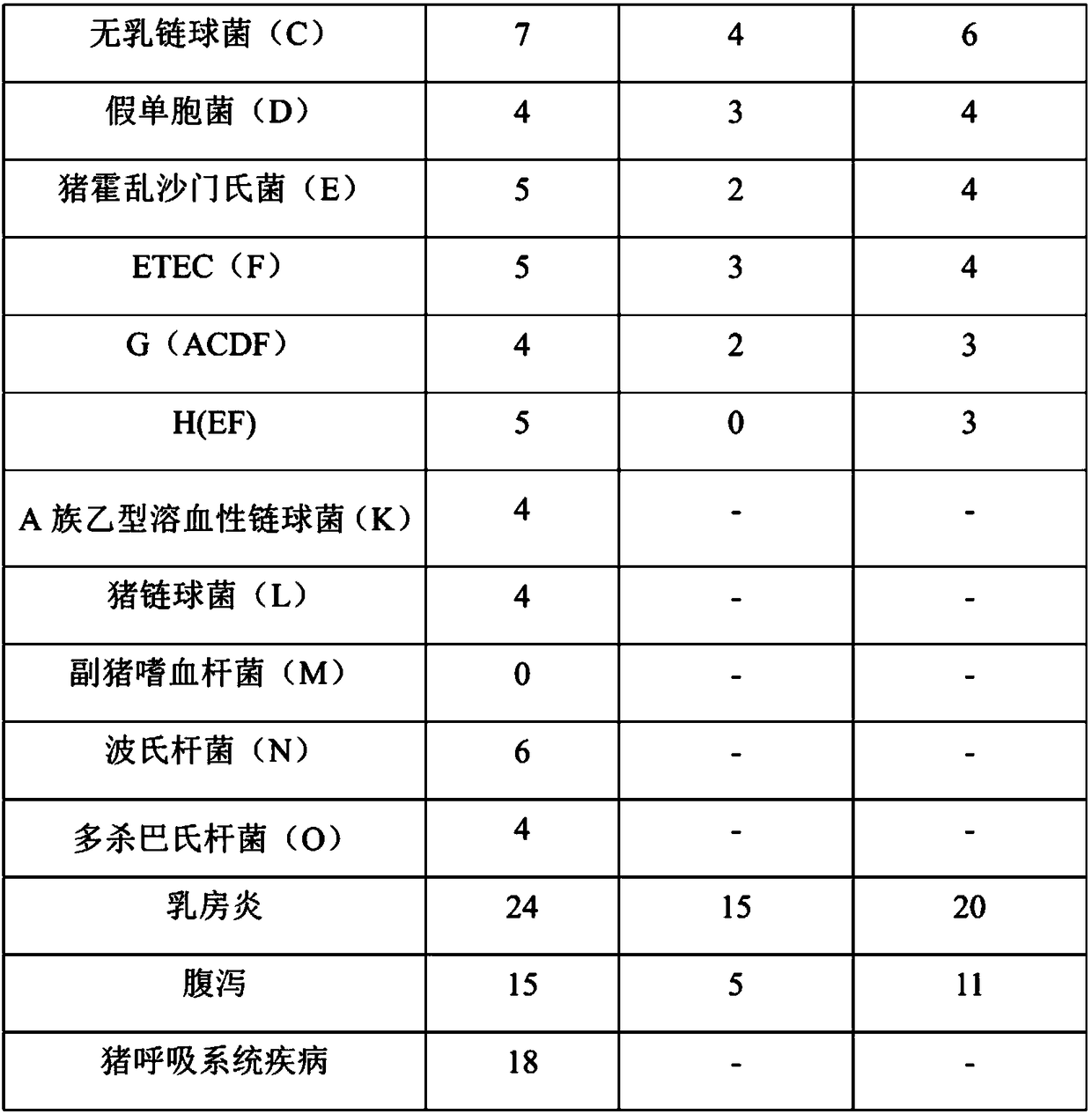
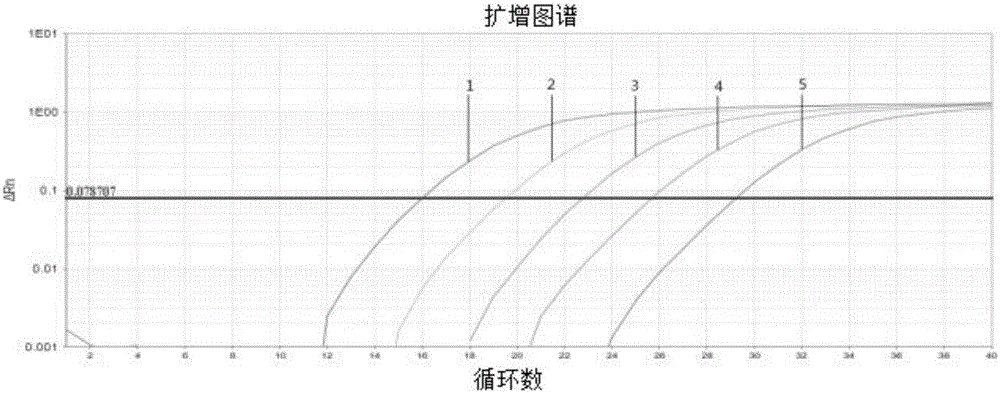
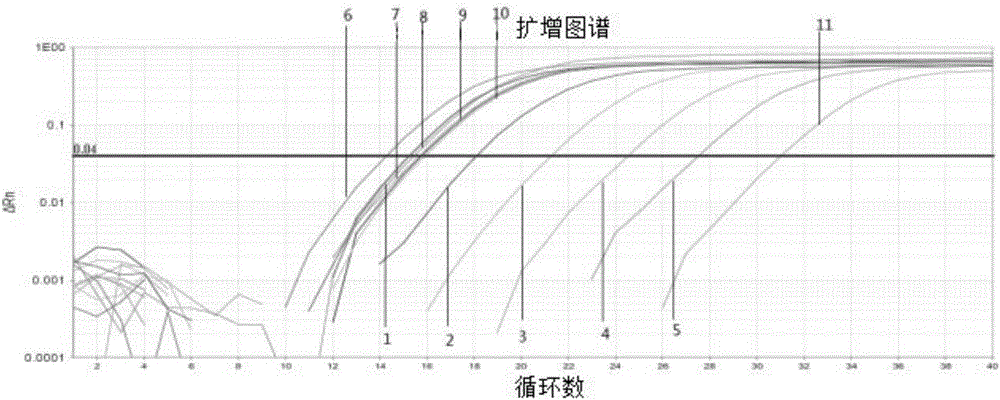
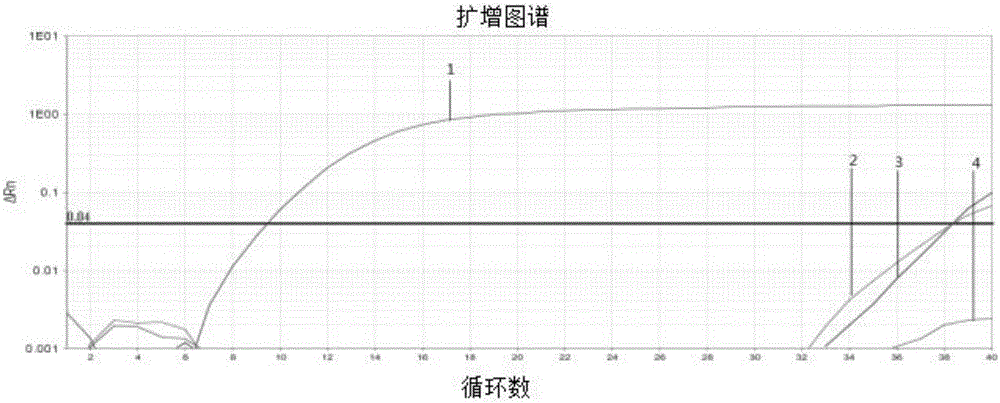
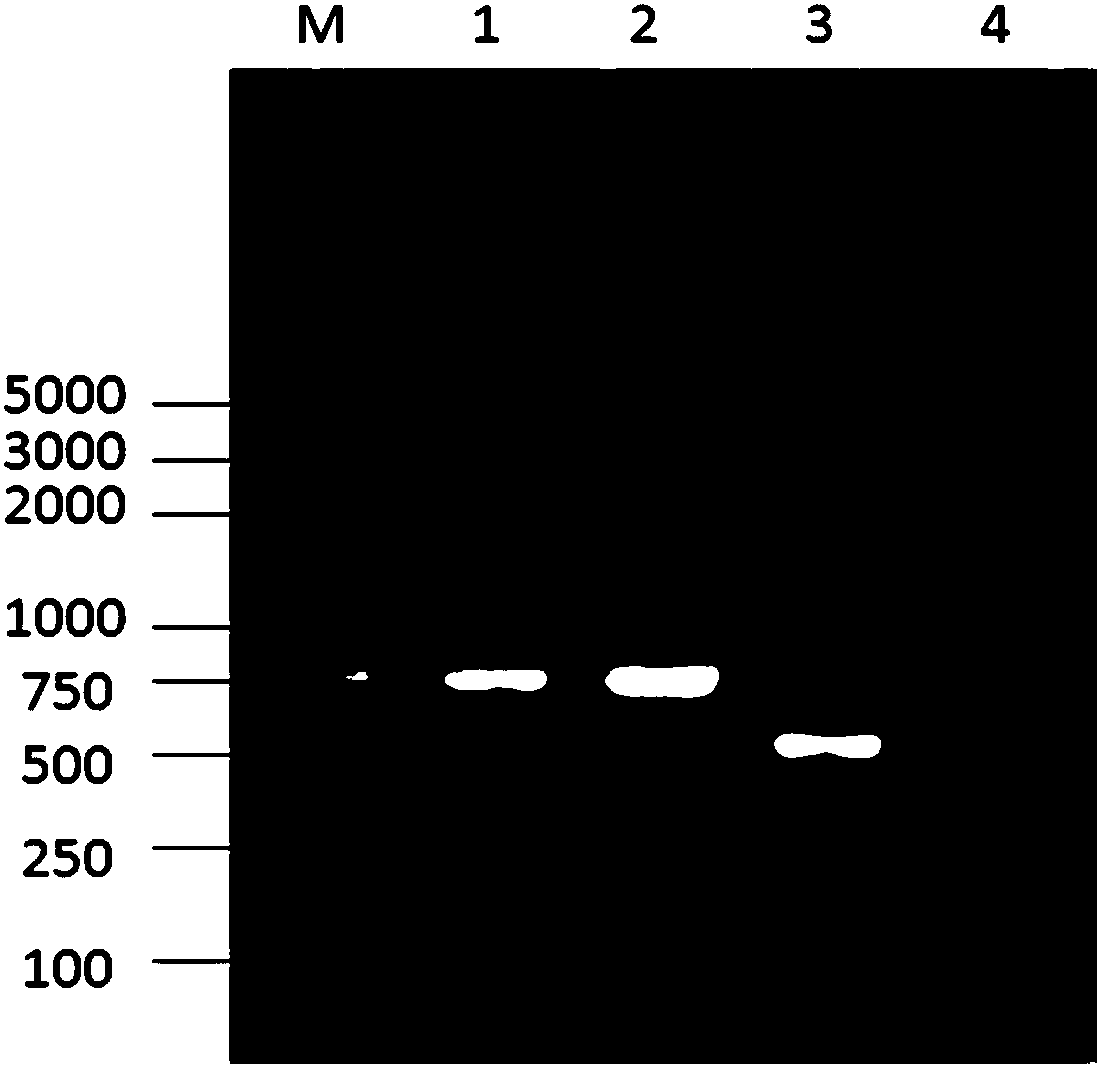
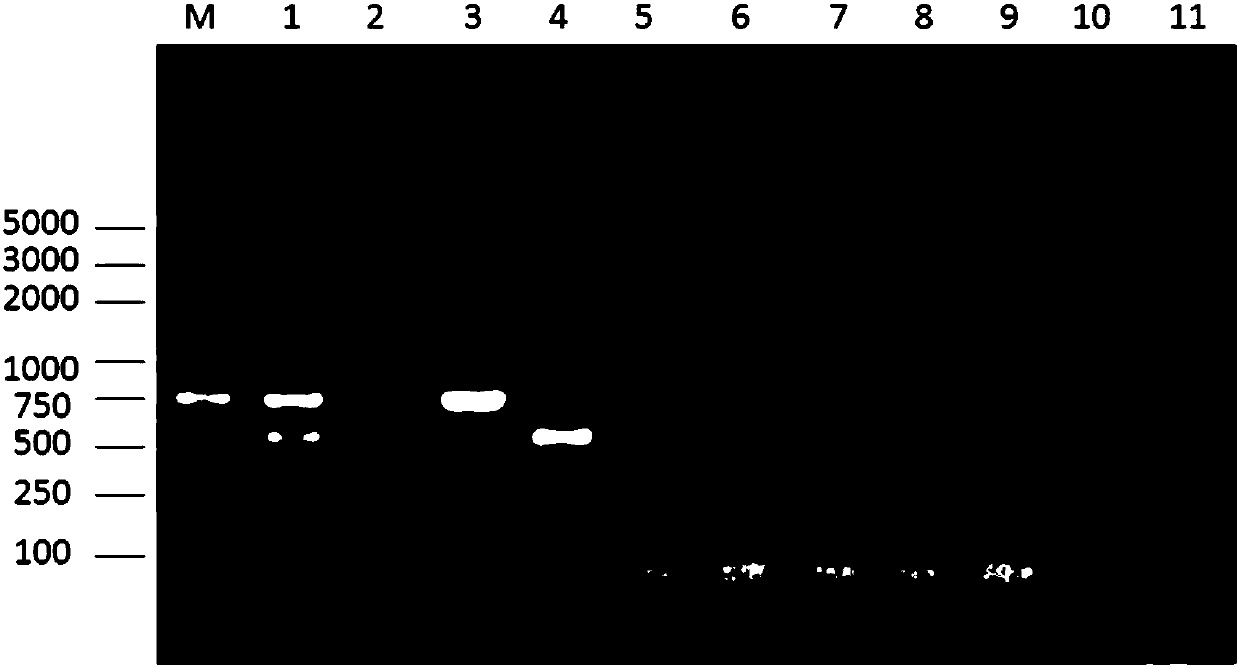
Patents
Literature
32 results about "Erysipelothrix rhusiopathiae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
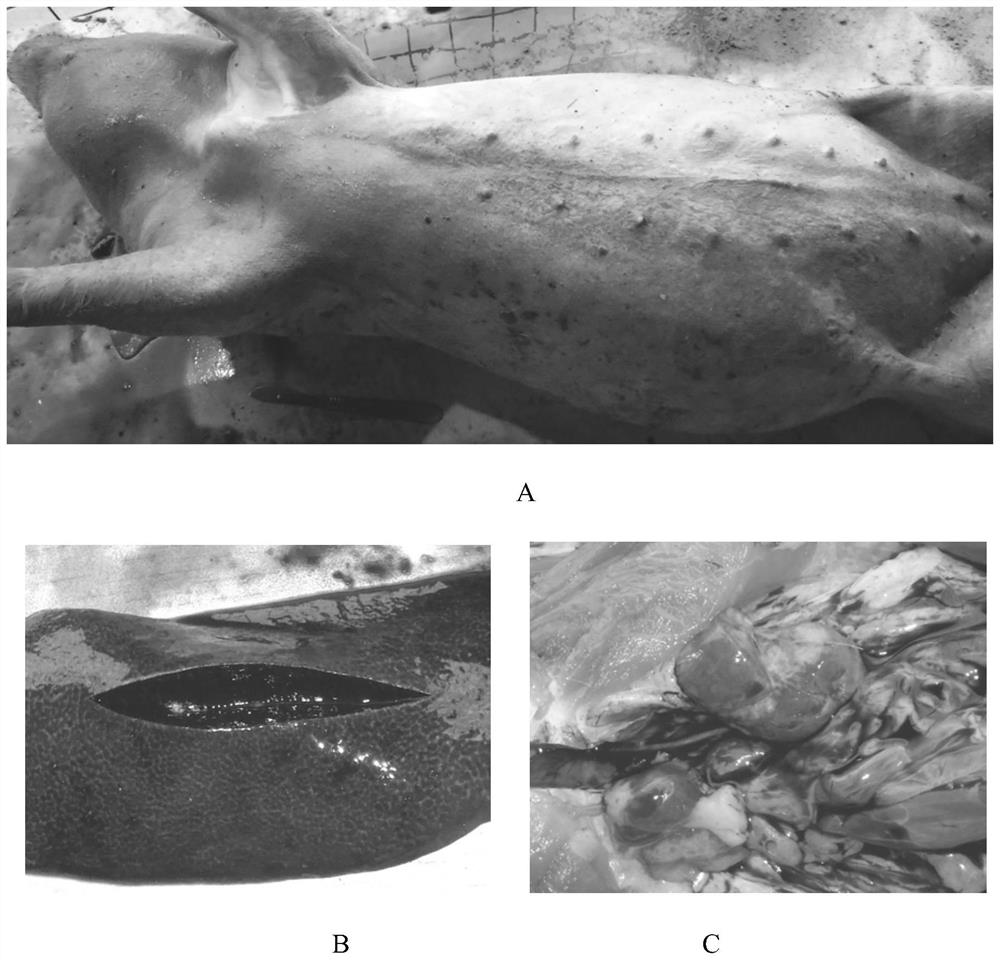
Erysipelothrix rhusiopathiae is a Gram-positive, catalase-negative, rod-shaped, non-spore-forming, nonacid-fast, nonmotile bacterium. Distributed worldwide, E. rhusiopathiae is primarily considered an animal pathogen, causing the disease known as erysipelas that may affect a wide range of animals. Pigs, turkeys and laying hens are most commonly affected, but cases have been reported in other mammals, birds, fish, and reptiles. In pigs, the disease is known as "diamond skin disease". The bacterium can also cause zoonotic infections in humans, called erysipeloid. The human disease called erysipelas is not caused by E. rhusiopathiae, but by various members of the genus Streptococcus.
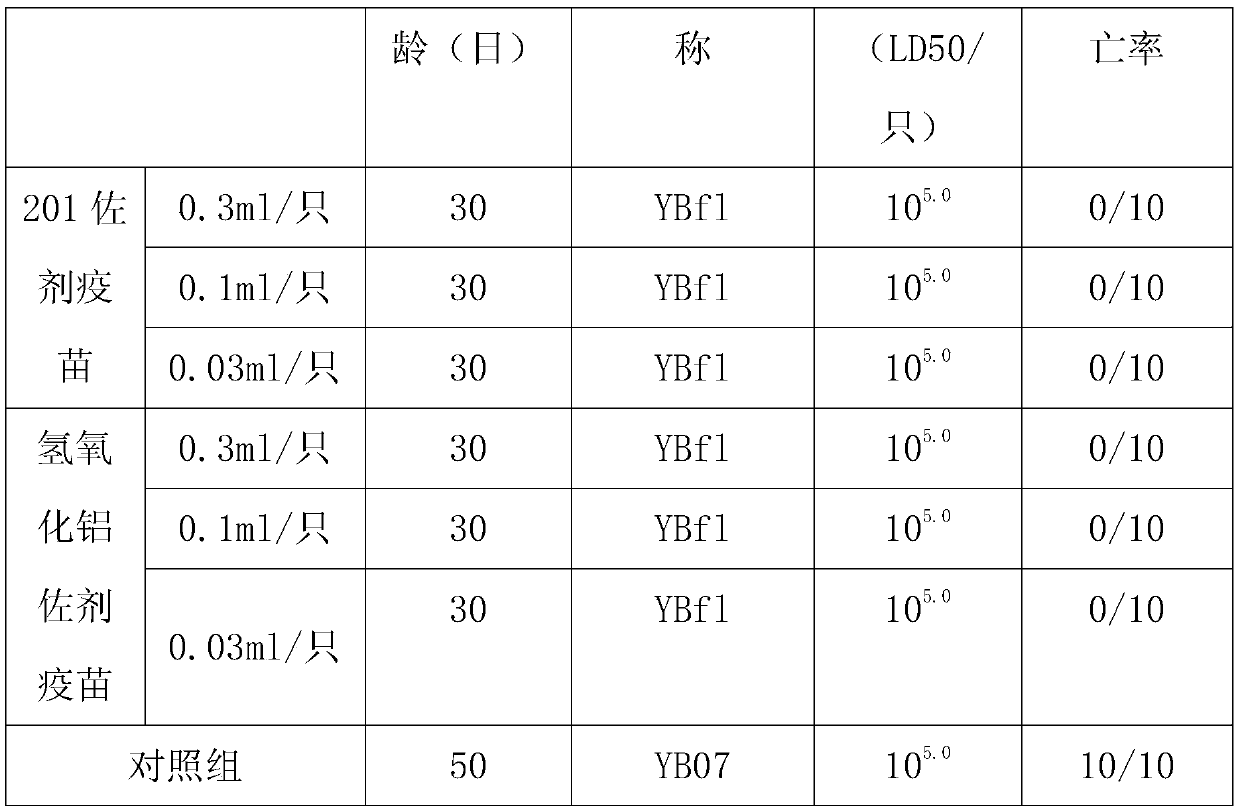
Process for producing erysipelothrix rhusiopathiae surface protective antigen mutant in escherichia coli
Owner:MEIJI ANIMAL HEALTH CO LTD
Novel antibacterial antiviral feed additive
InactiveCN102845607AImprove antibacterial propertiesLow inhibitory concentrationAntibacterial agentsAnthropod material medical ingredientsEscherichia coliMycotoxin
The invention discloses a novel antibacterial antiviral feed additive for a livestock and poultry breeding industry. The novel antibacterial antiviral feed additive is composed of plume poppy, lightyellow sophora root, macleaya microcarpa, yucconin, tea saponin, Origanum vulgare L., narcissus seed and pagodatree flower bud powder and also comprises a hydrophilic aluminosilicate as a carrier. The novel antibacterial antiviral feed additive has the advantages that specific active components of natural plants can directly or indirectly kill viruses, have strong antibacterial effects and a wide antibacterial spectrum and compared with the common drugs such as berberine hydrochloride, penicillin and aureomycin, the specific active components have stronger activities of resisting some bacteria; in feeding, antibiotic drugs are avoided and mycotoxins are reduced and good effects of treating and preventing avian pasteurellosis, escherichia coli, bacillus rhusiopathiae suis, cholera fowl, white scour of piglets, and pullorum disease are obtained; and the novel antibacterial antiviral feed additive can resist stress and coccidium and remove heavy metals and drug residues.
Owner:王茜 +1
LAMP kit for detection of bacillus erysipelatos-suis and detection method
InactiveCN105755143AAccurate identificationHigh sensitivityMicrobiological testing/measurementMicroorganism based processesField testsBuffer solution
The invention provides an LAMP kit for detection of bacillus erysipelatos-suis.The LAMP kit comprises a primer, a Bst DNA polymerase, a reaction buffer solution and nucleic acid dye.The invention further provides an LAMP method for detection of bacillus erysipelatos-suis.Through the LAMP kit or method, bacillus erysipelatos-suis can be authenticated and detected quickly and accurately, and the LAMP kit or method has the advantages of high specificity, short consumption time, high sensitivity, easy and convenient authentication and the like and is suitable for a laboratory or field tests.
Owner:WUHAN BAIYUAN TECH CO LTD
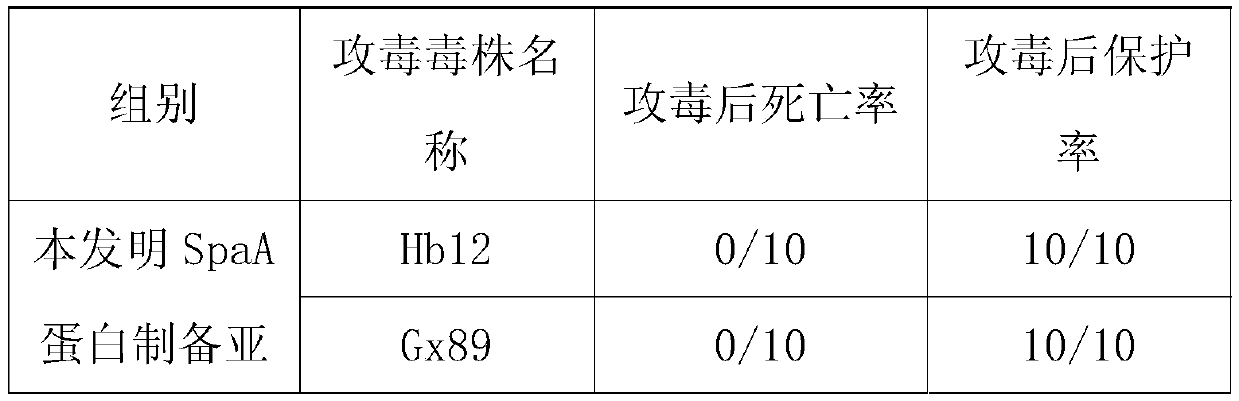
Erysipelothrix rhusiopathiae subunit vaccine, preparation method and application
ActiveCN106146626AImprove protectionPrevent invasionAntibacterial agentsBacterial antigen ingredientsEscherichia coliNucleotide
The invention provides an erysipelothrix rhusiopathiae subunit vaccine, a preparation method and application. The subunit vaccine contains an amino acid sequence shown as SEQ ID NO.2, the sequence is different from any disclosed related sequences, a nucleotide sequence corresponding to protein is cloned to escherichia coli, and then a genetically engineered bacterium-escherichia coli BL21 / pET-28a-spaA capable of expressing spaA protein is obtained, wherein the preservation number is CCTCC NO:M2015076. The genetically engineered bacterium is high in protein expression quantity, easy to operate and suitable for large-scale subunit vaccine production. The prepared vaccine is high in antibody level generated to an organism and long in continuity, has the safety and the stability and is the erysipelothrix rhusiopathiae subunit vaccine which extremely has development potential.
Owner:HUAZHONG AGRI UNIV +1
Pair of specific primers for detecting Bergeyella, kit and PCR detection method
PendingCN109055588ARapid identificationEasy to identifyMicrobiological testing/measurementAgainst vector-borne diseasesP. multocidaSpecific primers
The invention discloses a pair of specific primers for detecting Bergeyella, a kit and a PCR detection method. The primers comprise an upstream primer Bergeyella-F: 5'-TTGAAAGCTCCGGCGGATAG-3' and a downstream primer Bergeyella-R: 5'-ACCCTCACGAGAGTAGGTTT-3'. According to the specific primers, the kit and the PCR detection method, streptococcus, bacillus erysipelatos-suis, pasteurella multocida, salmonella, haemophilus parasuis, infectious actinobacillus pleuropneumoniae, stenotrophomonas maltophilia, pig Bergeyella and a Bergeyella zoohelcum 16S rRNA gene sequence are downloaded from a GenBankdatabase; sequence comparison is carried out through MEGA 5.05 software to search a Bergeyella 16S rRNA specific sequence; and Primer-BLAST design is utilized to amplify a pair of specific primers ofBergeyella; and a Bergeyella PCR rapid detection method is established to identify Bergeyella. The method has the advantages of being rapid, convenient, high in sensitivity and strong in specificity.
Owner:HENAN UNIV OF ANIMAL HUSBANDRY & ECONOMY
Swine erysipelas Spa A protein and application thereof to preparation of vaccines
ActiveCN110183520AHigh antigen stabilityHigh purityAntibacterial agentsBacterial antigen ingredientsAntigenTerra firma
The invention provides swine erysipelas Spa A protein. The amino acid sequence of the swine erysipelas Spa A protein is SEQ ID NO: 1. The erysipelothrix rhusiopathiae Spa A protein is used for preparing vaccines or diagnostic reagents. A subunit vaccine prepared from the erysipelothrix rhusiopathiae Spa A protein has the characteristics that antigen stability is high, purity is high, specificity is high, other uncorrelated antibodies are not generated, and the detection method is convenient and accurate, and a firm basis is established for industrial production of swine erysipelas subunit vaccines and diagnostic reagents.
Owner:YEBIO BIOENG OF QINGDAO
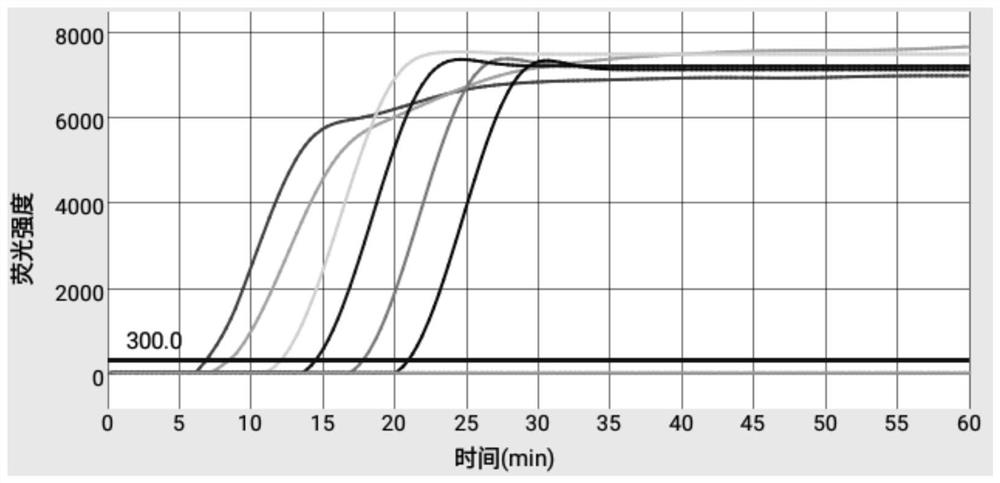
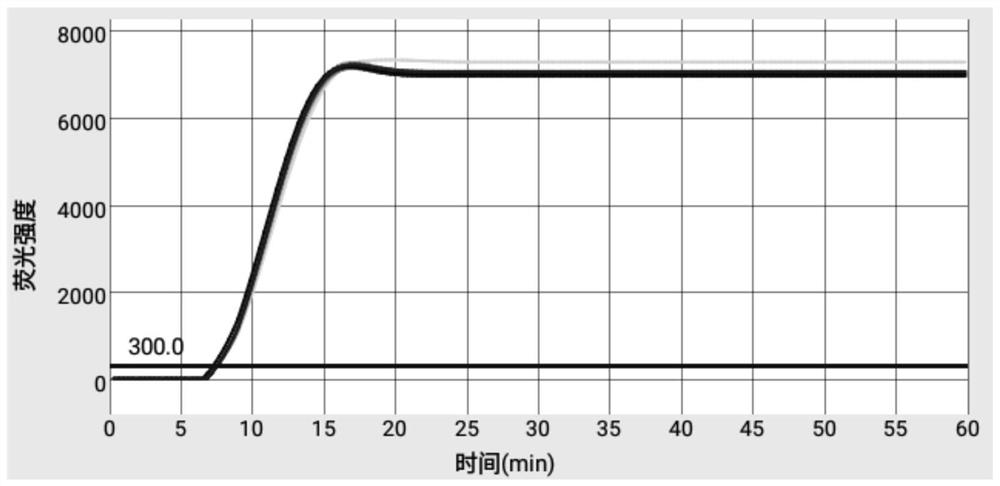
Primer and kit for efficiently detecting Erysipelothrix rhusiopathiae
PendingCN112029878ASimple and fast operationSuppresses non-specific reactionsMicrobiological testing/measurementDNA/RNA fragmentationSwine ErysipelasMedicine
The invention discloses a primer and kit for efficiently detecting Erysipelothrix rhusiopathiae. According to the primer and the kit, an improved LAMP technology serves as a gene amplification reaction principle, and gold nanoparticles are added into a reaction system to adsorb ssDNA and protease and inhibit nonspecific reactions during heating, thus, the aim of warm start is achieved, and the nonspecific reactions during heating are avoided; by combining the improved LAMP technology and a microfluidic chip technology, an accurate detection result can be presented rapidly, and the aim of jointdetection on the same sample with a plurality of different indexes is achieved; and meanwhile, reaction reagents are pre-buried into a microfluidic chip, a user only needs to add a sample, and operations are simple and convenient.
Owner:宁波爱基因科技有限公司
Uses of Phyllanthus emblica, and Phyllanthus emblica composition and uses thereof
InactiveCN109276602AExpand the scope of antibacterial applicationGrowth inhibitionAntibacterial agentsDigestive systemEscherichia coliBacteroides
The invention discloses applications of Phyllanthus emblica or Phyllanthus emblica and a Phyllanthus emblica composition in preparation of products for preventing and / or treating at least one of mastitis, diarrhea and porcine respiratory diseases, and further provides antibacterial effects of Phyllanthus emblica or Phyllanthus emblica extract and the Phyllanthus emblica composition, wherein the Phyllanthus emblica or Phyllanthus emblica extract and the Phyllanthus emblica composition can effectively inhibit the growth of one or a variety of bacteria selected from Staphylococcus aureus, Streptococcus agalactiae, Pseudomonas, Salmonella choleraesuis, Escherichia coli, Group A beta-hemolytic streptococcus, Streptococcus suis, Bordetella, Pasteurella multocida, Salmonella enterica, Staphylococcus haemolyticus, Enterococcus faecalis and Erysipelothrix rhusiopathiae, and the effectiveness of the uses are proved through experiments so as to expanded the applications of Phyllanthus emblica andthe Phyllanthus emblica composition.
Owner:SICHUAN ANIMAL SCI ACAD
Erysipelothrix rhusiopathiae antigens and vaccine compositions
The invention relates to stabilized antigen compositions of Erysipelothrix rhusiopathiae and vaccine formulations containing such antigen compositions. Antigens of the invention are effective in providing long-term protection against erysipelas in animals.
Owner:ZOETIS SERVICE LLC
Primer, probe, kit and method for detecting bacillus erysipelatos-suis by fluorescent quantitative PCR (Polymerase Chain Reaction)
InactiveCN106435007AQuick distinctionImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceDNA
The invention discloses a primer, a probe, a kit and a method for detecting bacillus erysipelatos-suis by a fluorescent quantitative PCR (Polymerase Chain Reaction). The primer is shown as SEQ ID NO: 1 and SEQ ID NO: 2; the probe is shown as SEQ ID NO: 3. The method disclosed by the invention takes DNA (Deoxyribonucleic Acid) of a sample to be detected as a template for carrying out a fluorescent quantitative PCR amplification reaction; fluorescent signals are collected to draw a curve. The primer and the probe for detecting the bacillus erysipelatos-suis have very high specificity and sensitivity, can be used for detecting the bacillus erysipelatos-suis and can also be used for detecting various types of clinical samples, so that a clinical bacillus erysipelatos-suis infection condition can be rapidly monitored; the operation is simple and practical.
Owner:WENS FOOD GRP CO LTD
Pig erysipelothrix rhusiopathiae antigen and vaccine composition thereof
InactiveCN1262129AAntibacterial agentsBacterial antigen ingredientsAntigenErysipelothrix rhusiopathiae Antigen
The invention relates to stabilized antigen compositions of Erysipelothrix rhusiopathiae and vaccine formulations containing such antigen compositions. Antigens of the invention are effective in providing long-term protection against erysipelas in animals.
Owner:ZOETIS SERVICE LLC
Rapid detection for duplex PCR primer pair of bacillus erysipelatos-suis and streptococcus suis, and kit, and method
PendingCN107937573AReduce testing costsShorten the timeMicrobiological testing/measurementMicroorganism based processesBacteroidesDuplex pcr
The invention discloses rapid detection for a duplex PCR primer pair of bacillus erysipelatos-suis and streptococcus suis, and a kit and a method. Through PCR amplification to DNA of a to-be-detectedsample, the kit can rapidly detect bacillus erysipelatos-suis and streptococcus suis in the sample. The duplex PCR is based on common PCR, allows a pair of primers to be added in the reaction system,and is used to detect single infection and mixed infection of the two kinds of bacteria in a clinic sample. Therefore, a duplex PCR detection kit and a detection method of the two kinds of pathogenicbacteria are established and have a great significance on fast accurate identification of pathogenic bacteria in a clinic sample and molecular epidemiological investigation on pathogenic bacteria.
Owner:HUAZHONG AGRI UNIV
1a-type erysipelothrix rhusiopathiae and application thereof
ActiveCN109609418AHigh proliferative titerStrong pathogenicityAntibacterial agentsBacterial antigen ingredientsDiseaseBacteroides
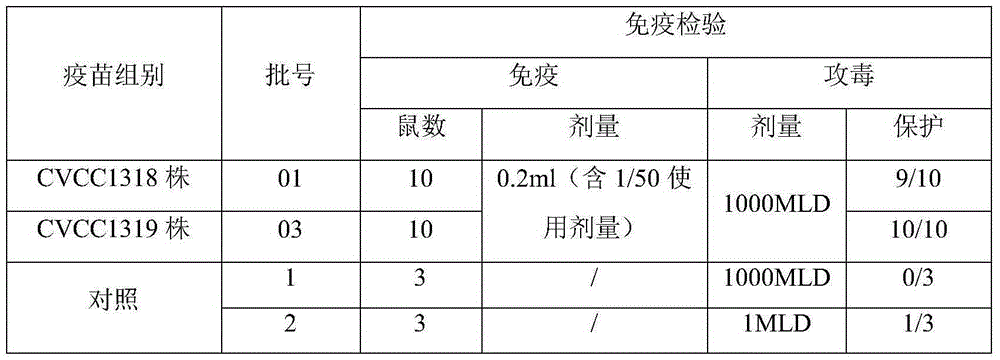
The invention discloses 1a-type erysipelothrix rhusiopathiae and an application thereof. The preservation number of the erysipelothrix rhusiopathiae is CGMCC NO:16808, names as erysipelothrix rhusiopathiae HN1573. The strain is separated from the heart blood of a fattening pig dying of acute sepsis. No other bacterium but the erysipelothrix rhusiopathiae grows during the separation on a TSA solidmedium when the proliferation titer in a TSB liquid medium is as high as 109 CFU / mL or above. A vaccine prepared by the strain HN1573 screened by the invention is simple in preparation process, and has good preventive effect on pig erysipelas disease.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
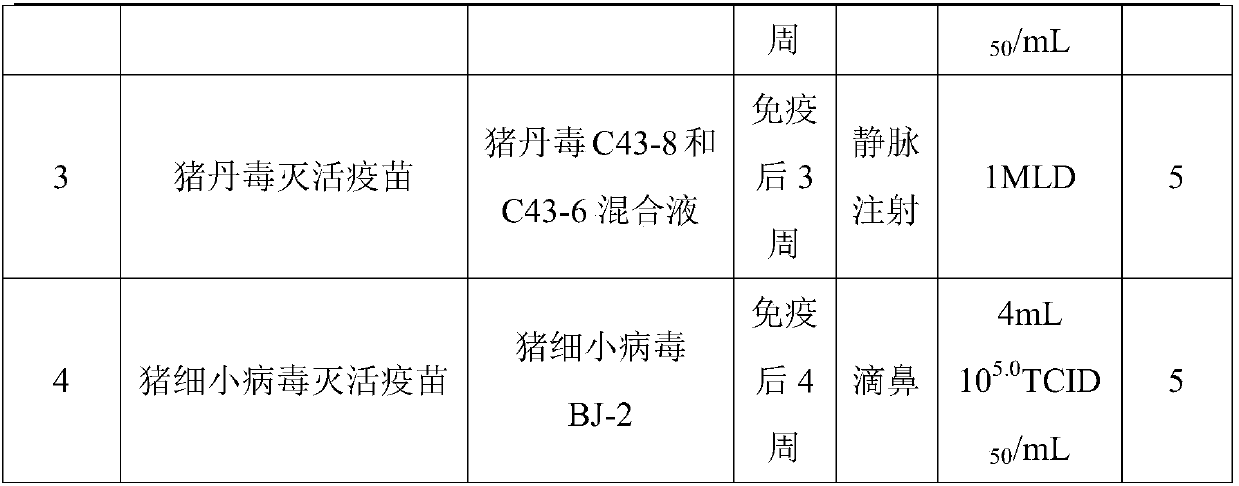
Method for preparing swine erysipelas and porcine parvovirus bivalent inactivated vaccine
InactiveCN107648599APlay the role of multiple defensesDoes not affect the mindAntibacterial agentsBacterial antigen ingredientsSwine ErysipelasImmunogenicity
The invention provides a method for preparing swine erysipelas and porcine parvovirus bivalent inactivated vaccine. The method has the advantages that bacteria solution is prepared from bacillus rhusiopathiae suis in fermentation modes in preparation procedures, a pH (potential of hydrogen) value of fermentation broth is strictly controlled in bacteria solution fermentation procedures, accordingly, the immunogenicity of bacillus rhusiopathiae suis antigens can be effectively guaranteed, and the protection rate can be higher than 90% without excessively high quantities of thalli of the bacillusrhusiopathiae suis; viruses can be reproduced from porcine parvovirus in IBRS-2 cell suspension culture modes by the aid of bioreactors in swine erysipelas and porcine parvovirus bivalent inactivatedvaccine preparation procedures, and accordingly the titer of virus liquid can be obviously improved; dissolved oxygen, pH, rotational speeds and the like are set in virus reproduction procedures, accordingly, the homogeneity of inter-assay virus liquid can be guaranteed, the shortcomings of complicated operation, high labor intensity, vulnerability to pollution and the like in spinner-bottle culture procedures can be overcome, and the quality stability of porcine parvovirus inactivated vaccine can be effectively enhanced.
Owner:TIANJIN RINGPU BIO TECH
Feed additive for preventing and treating viral disease of livestock and preparation method of feed additive
InactiveCN106721003AFunction increaseReduce mortalityAntibacterial agentsPowder deliveryEscherichia coliPenicillin
The invention discloses a novel antibacterial and anti-viral feed additive for livestock and poultry industry. The feed additive is prepared from corchorus capsularis L, radix sophorae flavescentis, macleaya cordata, ergosterol, tea saponin, origanum vulgare L, narcissus seeds and pagodatree flower bud flour. The feed additive is mainly characterized in that viruses are directly or indirectly inhibited and killed through specific active constituents of natural plants, the feed additive is high in antibacterial property and wide in antibacterial spectrum, and the activity on a few of bacteria is higher than that of common berberine hydrochloride, penicillin and aureomycin. An antibiotic drug can be completely avoided in the feeding process, mycotoxin is reduced, and the feed additive has good treatment and prevention effects on pastevula mulfocida, escherichia coli, bacillus erysipelatos-suis, cholera fowl, white scourofpiglets and pullorum disease. The food additive resists stress and is anticoccidial, and heavy metals and drug residues are eliminated.
Owner:新昌县柏克动物饲料技术开发有限公司
Traditional Chinese medicine applied to swine erysipelas and chicken brickpox
InactiveCN106176995AIncrease the variety of preventive drugsSuit one's needsAmphibian material medical ingredientsAntibacterial agentsDiseaseGround beetle
The invention discloses traditional Chinese medicine applied to swine erysipelas and chicken brickpox. The traditional Chinese medicine is prepared from the following traditional Chinese medicine raw materials in parts by weight: 20 to 35 parts of ground beetles, 30 to 45 parts of radix codonopsis, 40 to 60 parts of radix isatidis, 40 to 60 parts of radix et rhizoma rhei, 10 to 30 parts of climbing beans, 40 to 60 parts of herba artemisiae scopariae, 5 to 10 parts of scolopendra, 60 to 80 parts of dried roots of radix scutellariae and 8 to 15 parts of toad skin. The traditional Chinese medicine applied to swine erysipelas and chicken brickpox prepared by the invention aims at an acute febrile infectious disease caused by bacillus erysipelatos-suis; the disease has the main features of acute sepsis, hyperpyrexia, (subacute) skin rash, chronic verrucous endocarditis, cutaneous necrosis and chronic multiple non-suppurative arthritis; the effects of fast effect taking, no recurrence and disease condition control are achieved; and in addition, a direct treatment effect is also achieved on chicken brickpox attacked chicken groups with the symptoms of limp bodies, whitewater faeces, transfixed standing, full liquid filling in the crop, empurpled cockscomb, transfixed standing away from the group, falling wings and fluffy feathers.
Owner:鄢海军
Feed additive for preventing and treating swine erysipelas
InactiveCN106260670AImprove the immunityGreat tasteAnimal feeding stuffAccessory food factorsDiseaseFood additive
The invention relates to the technical field of aquaculture, and discloses a feed additive for preventing and treating swine erysipelas. The feed additive is prepared from, codonopsis pilosula, rhizoma polygonati, polygonatum odoratum, sanguisorba officinalis, agrimonia pilosa, nabalus, geum, erose mussaenda leaf, cudrania cochinchinensis, thunbergia grandiflora, glabrous sarcandra herb, marsdenia tenacissima, eggshell powder, mirabilitum praeparatum, black soybean powder, dietary alkali, aspergillus oryzae, bacillus natto, lycopene and compound enzyme. The feed additive does not have any toxic reagents and antibiotics, cannot generate residue and is remarkable in preventing and treating efficiency, the occurrence rate of the swine erysipelas is reduced to 0-1%, enzymolysis of various Chinese herbal medicines is achieved, tissue cell structures are destroyed, the Chinese herbal medicines are doubly fermented, palatability is improved, effective components are effectively extracted, the extracting rate reaches 86-88%, the use ratio of the Chinese herbal medicines is increased, the dietary alkali and the lycopene are added, pH(potential of hydrogen) and disease-resistant components of the feed additive are improved, activities of swine erysipelas bacilli are restrained, resistance of pigs is improved, and the swine erysipelas and other diseases are fundamentally restrained, so that aquaculture cost is reduced by 13.6%.
Owner:ANHUI HUAAO BIOTECH
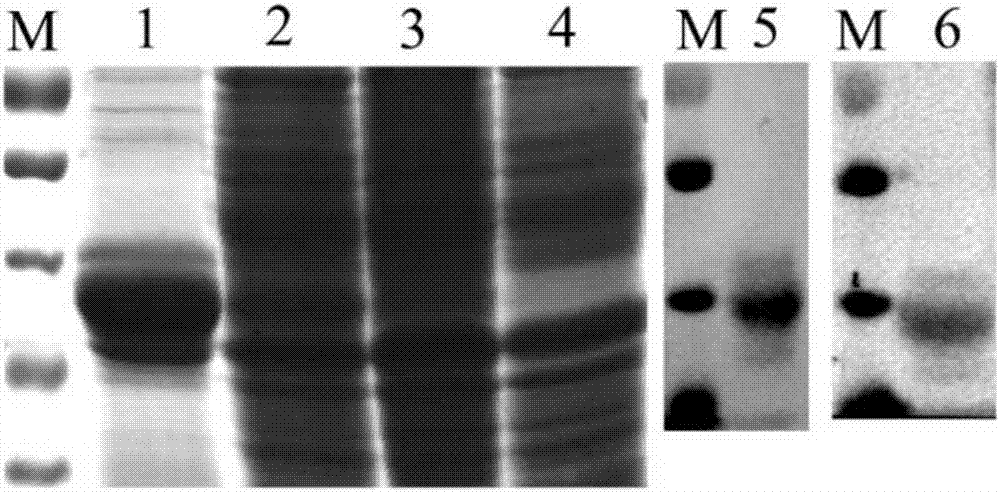
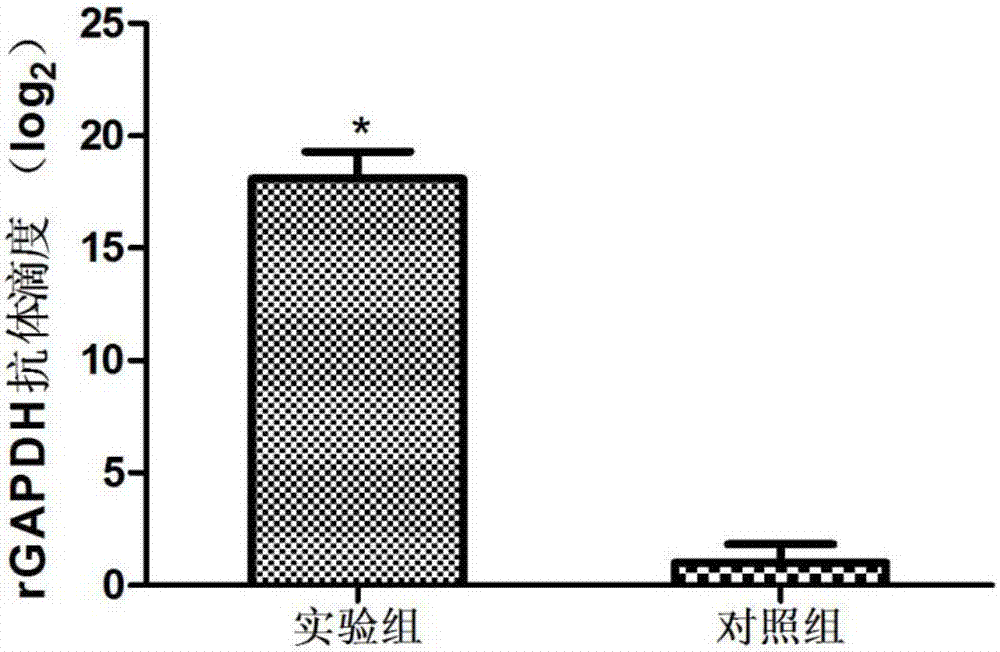
Gene gapdh for expressing erysipelothrix rhusiopathiae recombinant protein GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as well as recombinant escherichia coli and applications of gene gapdh
InactiveCN107974458AImprove survival rateAntibacterial agentsBacterial antigen ingredientsEscherichia coliAntigen
The invention discloses a gene gapdh for expressing erysipelothrix rhusiopathiae recombinant protein GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as well as recombinant escherichia coli and applications of the gene gapdh. According to the technical scheme, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene of erysipelothrix rhusiopathiae is cloned, expressed and purified. The western blot analysis for the porcine erysipelas rehabilitation pig serum shows that the recombinant protein GAPDH has very good immunogenicity. The mouse experiment shows that the recombinant protein GAPDH caninduce generation of high-titer antibodies and generate effective protection force. A novel candidate antigen is provided for the creation of a novel porcine erysipelas vaccine.
Owner:HUAZHONG AGRI UNIV
Culture medium for Erysipelothrix rhusiopathiae
InactiveCN106754455AIncrease proliferation rateGood training effectBacteriaMicroorganism based processesRehmannia glutinosaAngelica dahurica
The present invention provides a culture medium for Erysipelothrix rhusiopathiae, wherein the culture medium comprises, by weight, 8 parts of cortex meliae, 6 parts of wheat-middlings, 2 parts of beef extract, 1 part of maltose, 1 part of angelica dahurica, 1 part of black bean powder, and 1 part of serum, and on the basis, a specific amount of cortex phellodendri, rehmannia glutinosa or myristica fragrans houtt is further added. According to the present invention, a certain Chinese herb component is added on the basis of the commonly used materials of the culture medium, such that the unexpected prominent culture effect is obtained, and the obtained culture medium is especially suitable for the Erysipelothrix rhusiopathiae culture, and has god application prospects.
Owner:鼎正生物药业(天津)有限公司
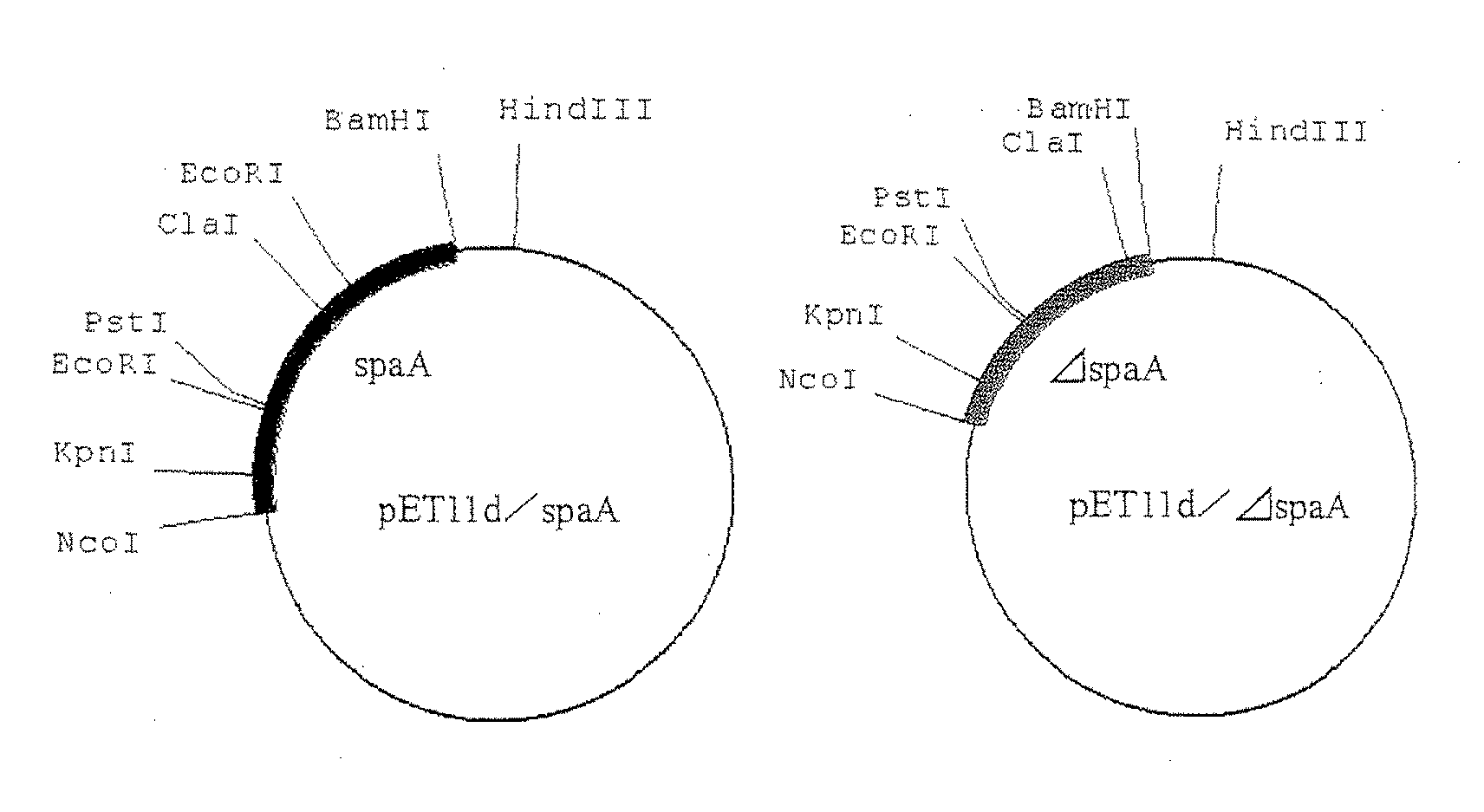
Process for preparing variant of erysipelothrix rhusiopathiae surface protective antigen in e. coli
ActiveUS20110059021A1Promote recoveryEasy to purifyAntibacterial agentsPeptide/protein ingredientsEscherichia coliProtective antigen
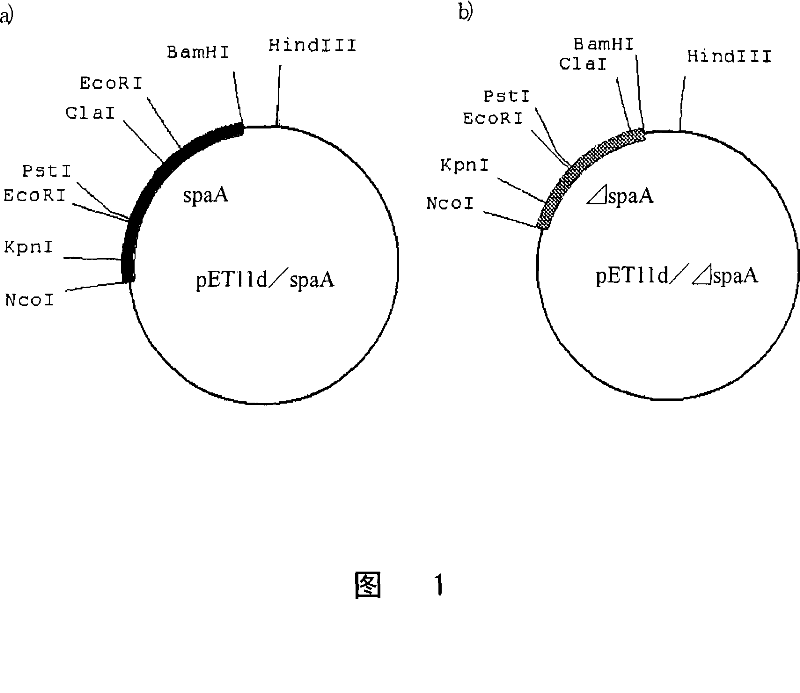
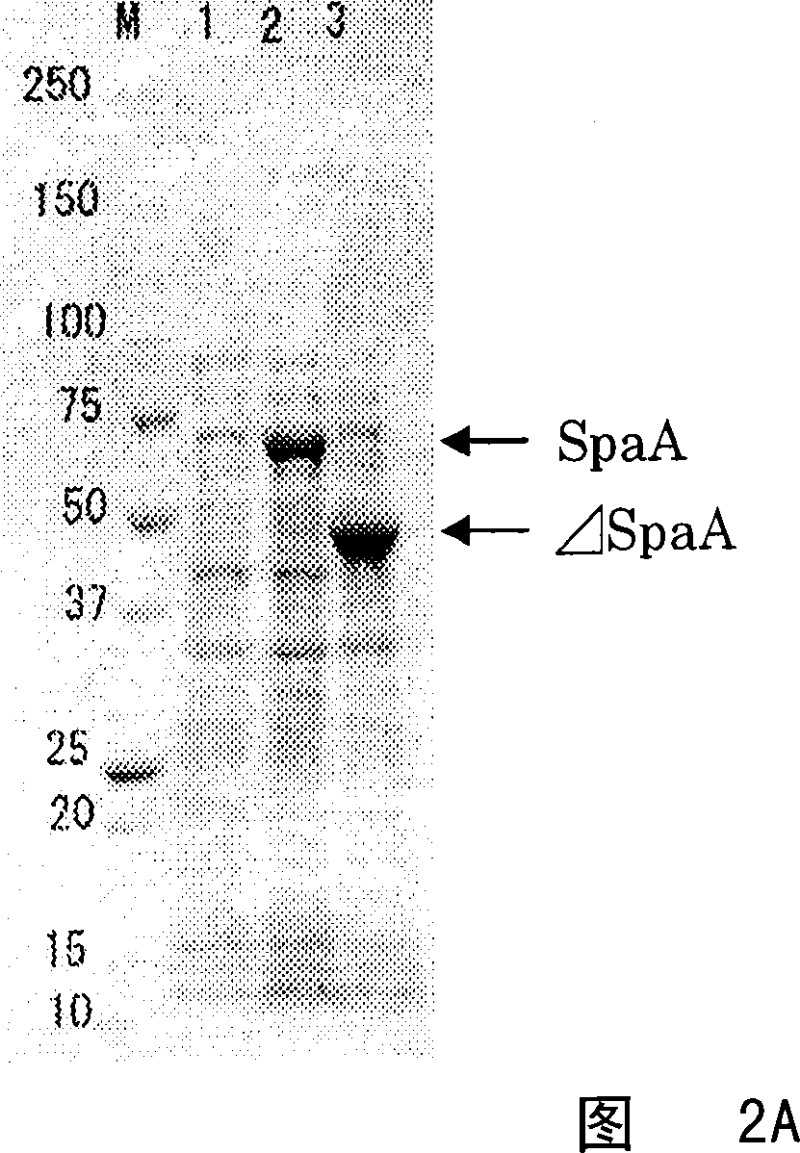
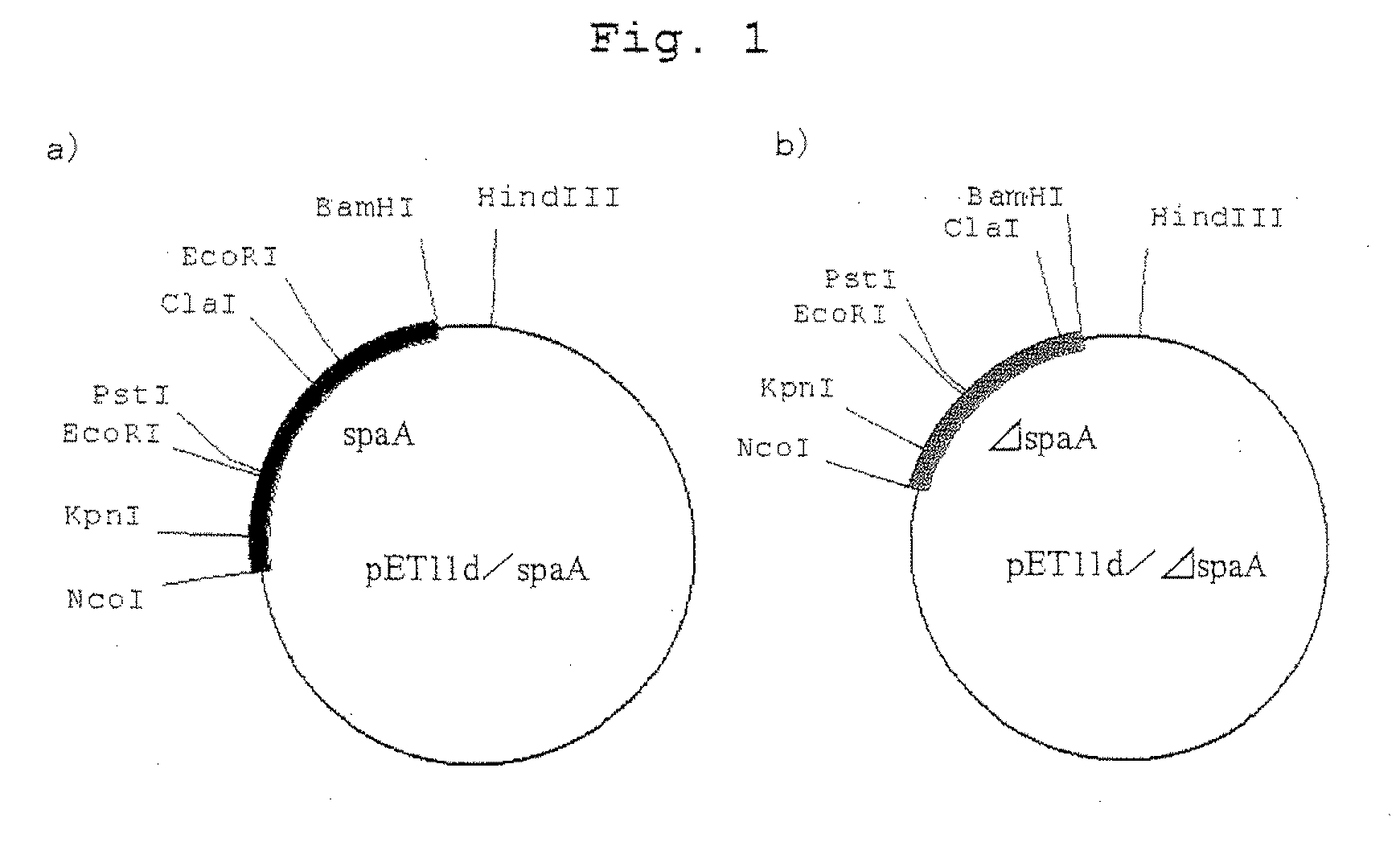
A variant of Erysipelothrix rhusiopathiae surface protective antigen SpaA protein or of a shortened form of SpaA (ΔSpaA) in which a portion of SpaA protein is deleted for protection from Erysipelothrix rhusiopathiae infection and a process for preparing the same are provided. Introduction of amino acid substitution at a specific site in the amino acid sequence of SpaA or ΔSpaA protein provides a variant of SpaA or ΔSpaA protein which is immunogenic and is expressed in E. coli as inclusion bodies. The variant of SpaA or ΔSpaA protein of the present invention may easily be recovered and purified since it is expressed in E. coli as inclusion bodies.
Owner:MEIJI ANIMAL HEALTH CO LTD
Bacillus erysipelatos-suis PCR (Polymerase Chain Reaction) primer and application thereof
ActiveCN103397023BIncreased sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationSwine ErysipelasAttenuated vaccine
The invention discloses a Bacillus erysipelatos-suis PCR (Polymerase Chain Reaction) primer and an application thereof, belonging to the fields of molecular biology and production of animal medicines. The nucleotide sequence of the Bacillus erysipelatos-suis PCR (Polymerase Chain Reaction) primer is shown in SEQIDNO:1-2. The Bacillus erysipelatos-suis PCR (Polymerase Chain Reaction) primer in the invention can be used to prepare a detection reagent of Bacillus erysipelatos-suis, can distinguish swine erysipelas attenuated vaccine strain G4T10 strain and other wild prevalent strains, and is contributed to monitoring the epidemic prevention condition of vaccines and diagnosing swine erysipelas condition in production.
Owner:WENS FOOD GRP CO LTD
Process for Preparing Variant of Erysipelothrix Rhusiopathiae Surface Protective Antigen in E. Coli
ActiveUS20080286309A1Sufficient immunogenicityAvoid insufficient purityAntibacterial agentsPeptide/protein ingredientsEscherichia coliProtective antigen
A variant of Erysipelothrix rhusiopathiae surface protective antigen SpaA protein or of a shortened form of SpaA (ΔSpaA) in which a portion of SpaA protein is deleted for protection from Erysipelothrix rhusiopathiae infection and a process for preparing the same are provided. Introduction of amino acid substitution at a specific site in the amino acid sequence of SpaA or ΔSpaA protein provides a variant of SpaA or ΔSpaA protein which is immunogenic and is expressed in E. coli as inclusion bodies. The variant of SpaA or ΔSpaA protein of the present invention may easily be recovered and purified since it is expressed in E. coli as inclusion bodies.
Owner:MEIJI ANIMAL HEALTH CO LTD
Erysipelothrix rhusiopathiae-haemophilus parasuis vaccine and methods of using the same
ActiveUS8637047B2Effective immunityReduce development riskAntibacterial agentsBacterial antigen ingredientsImmunopotencyAdjuvant
The present invention provides a composition and an improved single dose vaccine against E. rhusiopathiae and an improved single dose vaccine against E. rhusiopathiae and H. parasuis which provides one or more of the following: 1) confers effective immunity against E. rhusiopathiae and / or H. parasuis; 2) decreases the risk of developing clinical signs of E. rhusiopathiae and / or H. parasuis infection; 3) induces an immune response against E. rhusiopathiae and / or H. parasuis; and 4) has a DOI against E. rhusiopathiae and / or H. parasuis of at least four months. The composition or E. rhusiopathiae vaccine as well as the combined E. rhusiopathiae-H. parasuis composition or vaccine each includes a bacterial component of inactivated E. rhusiopathiae bacteria and a suitable adjuvant. The combined E. rhusiopathiae-H. parasuis composition or vaccine further includes an amount of H. parasuis antigen. The vaccines can be administered to animals in any conventional manner. The amount of the dose for intramuscular administration is preferably less than 5 ml. The amount of E. rhusiopathiae and / or H. parasuis antigen in each dose should be enough to induce an immune response in the animal receiving the vaccine or composition and will preferably confer effective immunity against and decrease the risk of developing clinical signs resulting from E. rhusiopathiae and / or H. parasuis infection for a suitable duration of immunity.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
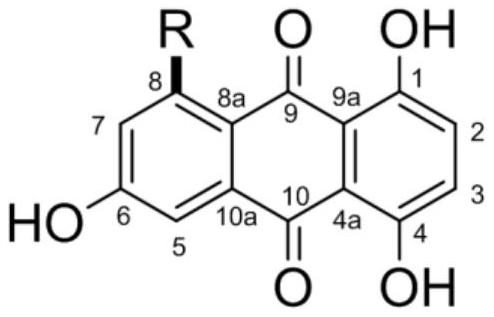
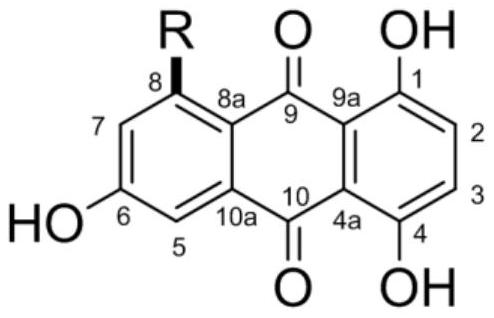
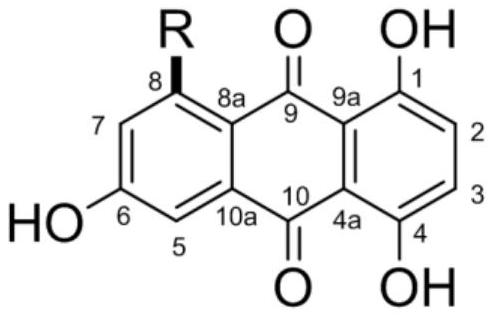
1,4,6-trihydroxy-8-branched-chain-9,10-anthraquinone compound and application thereof in preparation of bacteriostatic agent
PendingCN113912484AAnti-Staphylococcus aureusStreptococcus suisAntibacterial agentsOrganic chemistrySwine ErysipelasVeterinary Drugs
The invention relates to the technical field of pharmaceutical chemistry, and particularly discloses a 1,4,6-trihydroxy-8-branched-chain-9,10-anthraquinone compound and application thereof in preparation of a bacteriostatic agent. Antibacterial activity tests show that the 1,4,6-trihydroxy-8-branched-chain-9,10-anthraquinone compound can significantly inhibit growth of Staphylococcus aureus, Erysipelothrix rhusiopathiae and Streptococcus suis, and the compound can be used for preparing novel antibacterial veterinary drugs.
Owner:HUBEI BIOPESTICIDE ENG RES CENT
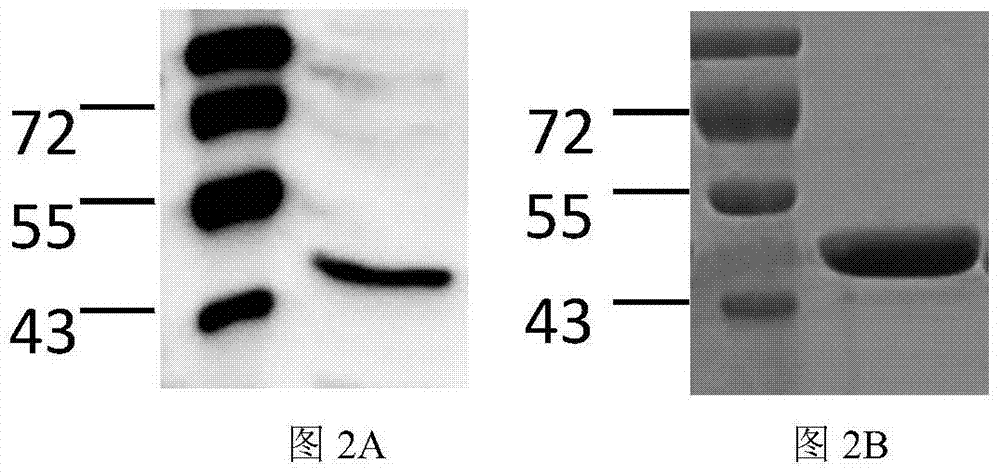
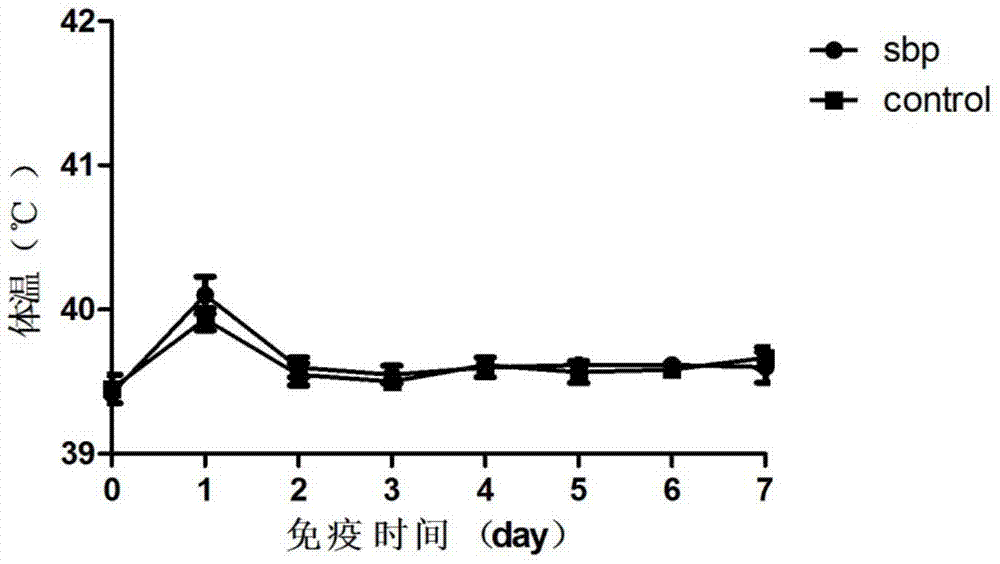
Erysipelothrix rhusiopathiae antigen protein sbp and application
ActiveCN107033227AImprove protectionHigh biosecurityAntibacterial agentsBacterial antigen ingredientsBiotechnologyErysipelothrix rhusiopathiae Antigen
The invention discloses erysipelothrix rhusiopathiae antigen protein sbp and an application. The amino acid sequence of the protein sbp is shown in SEQ ID NO.2. The protein is mixed with an adjuvant in proportion after aseptic treatment, piglets are immunized intramuscularly, it is found that the protein can effectively increase the survival rate of the piglets after challenge and increase the recovery speed of the piglets, and safety tests prove that immunized piglets have no adverse effects; meanwhile, the corresponding nucleotide sequence of the protein is compared with the sequences of 31 erysipelothrix rhusiopathiae, and the homology is found to be higher than or equal to 98%. Therefore, the antigen protein is highly conservative in different clinical isolates and can protect pig groups against attack of the current epidemic strains.
Owner:HUAZHONG AGRI UNIV
Erysipelothrix rhusiopathiae-haemophilus parasuis vaccine and methods of using the same
ActiveUS20060093622A1Reduce riskEffective immunityAntibacterial agentsBacterial antigen ingredientsAntigenAdjuvant
The present invention provides a composition and an improved single dose vaccine against E. rhusiopathiae and an improved single dose vaccine against E. rhusiopathiae and H. parasuis which provides one or more of the following: 1) confers effective immunity against E. rhusiopathiae and / or H. parasuis; 2) decreases the risk of developing clinical signs of E. rhusiopathiae and / or H. parasuis infection; 3) induces an immune response against E. rhusiopathiae and / or H. parasuis; and 4) has a DOI against E. rhusiopathiae and / or H. parasuis of at least four months. The composition or E. rhusiopathiae vaccine as well as the combined E. rhusiopathiae-H. parasuis composition or vaccine each includes a bacterial component of inactivated E. rhusiopathiae bacteria and a suitable adjuvant. The combined E. rhusiopathiae-H. parasuis composition or vaccine further includes an amount of H. parasuis antigen. The vaccines can be administered to animals in any conventional manner. The amount of the dose for intramuscular administration is preferably less than 5 ml. The amount of E. rhusiopathiae and / or H. parasuis antigen in each dose should be enough to induce an immune response in the animal receiving the vaccine or composition and will preferably confer effective immunity against and decrease the risk of developing clinical signs resulting from E. rhusiopathiae and / or H. parasuis infection for a suitable duration of immunity.
Owner:BOEHRINGER INGELHEM VETMADICA INC
A kind of production method of porcine erysipelas live vaccine
ActiveCN104306963BSource controllableHigh activityAntibacterial agentsBacterial antigen ingredientsHigh cellFreeze-drying
The invention relates to application of a synthetic medium and a freeze-drying protective agent in the production of a swine erysipelas live vaccine. The synthetic medium provided by the invention is designed according to growth characteristics and growth requirements of bacillus erysipelatos-suis, is controllable in raw material sources of the formula, is not influenced by materials such as beef, beef liver, pig stomach and so on, is stable in batch, simple in manufacturing, convenient to use, suitable for carrying out high cell density culture of bacteria and high in amount of culture bacteria; in the freeze-drying process, through the adoption of the freeze-drying protective agent developed for the characteristics of the bacillus erysipelatos-suis and a matched freeze-drying curve, the activity of the bacteria is effectively protected in the freeze-drying process, so that the survive rate of the freeze-dried bacteria is increased and the vaccine is preserved for a long time at 2-8 DEG C and can be preserved for 24 months.
Owner:北京中海生物科技有限公司 +1
Indirect ELISA kit for detecting various animal erysipelothrix rhusiopathiae antibody, preparation method, and application of indirect ELISA kit
InactiveCN107664699AHigh activityReduce concentrationBiological testingPositive controlHorse radish peroxidase
The invention relates to erysipelothrix rhusiopathiae (a bacterial strain number: YN15075, and a bacterial strain preservation number: CGMCC No.14468) and an antibody indirect ELISA detection kit, a preparation method and application. The kit is prepared by assembling an elisa plate, a serologecal plate, a coating antigen, a coating buffer solution, positive control serum A, positive control serumB, negative control serum, horse radish peroxidase marked staphylococcus protein A (HRP-SPA), degreased milk powder, a 100*TMB substrate solution, 10*developing diluents, a 3 percent (v / v) H2O2 solution, a stop solution, and a 25*washing buffer solution. The kit can detect erysipelothrix rhusiopathiae antibodies of pigs, cattle, sheep and rabbits; a result judgment standard is as follows: on thepremise of ensuring the effectiveness of the test, the positive standard of the pig, cattle, sheep and rabbit erysipelothrix rhusiopathiae antibody is separately that S / P is greater than or equal to 0.30, S / P is greater than or equal to 0.28, S / P is greater than or equal to 0.26, and S / P is greater than or equal to 0.33 (S indicates a OD450nm value of the detected serum, and P indicates an averagevalue of the OD450nm value of the 2-pore positive control serum), otherwise, the result is determined as negative; the kit can be used for the erysipelothrix rhusiopathiae antibody detection and epidemiology investigation.
Owner:YUNNAN ANIMAL SCI & VETERINARY INST
A kit and detection method for detecting bacteria
InactiveCN105755143BAccurate identificationHigh sensitivityMicrobiological testing/measurementMicroorganism based processesField testsBuffer solution
The invention provides an LAMP kit for detection of bacillus erysipelatos-suis.The LAMP kit comprises a primer, a Bst DNA polymerase, a reaction buffer solution and nucleic acid dye.The invention further provides an LAMP method for detection of bacillus erysipelatos-suis.Through the LAMP kit or method, bacillus erysipelatos-suis can be authenticated and detected quickly and accurately, and the LAMP kit or method has the advantages of high specificity, short consumption time, high sensitivity, easy and convenient authentication and the like and is suitable for a laboratory or field tests.
Owner:WUHAN BAIYUAN TECH CO LTD
Erysipelothrix rhusiopathiae and application thereof
PendingCN113897315AImprove protectionSignificant technological progressAntibacterial agentsBacterial antigen ingredientsErysipelothrix erysipeloidesSwine Erysipelas
The invention provides erysipelothrix rhusiopathiae. The preservation number of the erysipelothrix rhusiopathiae is CGMCC No: 23390. The invention further provides a vaccine. The vaccine is characterized in by containing inactivated erysipelothrix rhusiopathiae with the preservation number of CGMCC No: 23390. According to the erysipelothrix rhusiopathiae, a plurality of groups of clinically separated strains are screened, and an attacking protection experiment is carried out together with a traditional swine erysipelas vaccine, so that a vaccine candidate strain which has good protection effect on epidemic type 1 and type 2 strains in swine herds in China is found.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com