1a-type erysipelothrix rhusiopathiae and application thereof
A technology of swine erysipelas and toxins, applied in the direction of bacteria, antibacterial drugs, veterinary vaccines, etc., to achieve strong pathogenicity, good immunogenicity, and good protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Isolation and identification of strain HN1573
[0024] 1.1 Preparation of medium
[0025] TSB liquid medium: Dissolve 30g of TSB broth powder (Tryptic Soy broth, tryptone soybean broth) in 1000mL of ultrapure water, sterilize at 115°C for 15min, add 50mL of fetal bovine serum;
[0026] TSA solid medium: Dissolve 40 g of TSA agar powder (Tryptic Soy Agar, tryptone soybean agar) in 1000 mL of ultrapure water, sterilize at 115 °C for 15 min, add 50 mL of fetal bovine serum; store at 4 °C for later use.
[0027] 1.2 Isolation and cultivation of strains
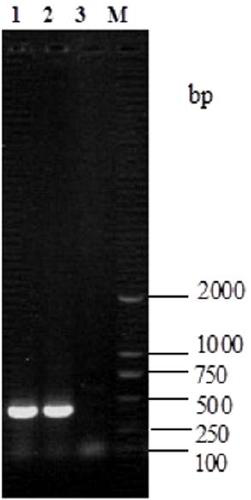
[0028] Aseptically collected heart blood from diseased pigs was inoculated on TSA solid medium, and cultured in a constant temperature incubator at 37°C for 24-36 hours, and the colonies with uniform needlepoint size, colorless and transparent, round, smooth and with neat edges grew out (see figure 1 ). After purification and inoculation, carry out Gram staining, the bacteria are Gram-positive bacteria, straig...
Embodiment 2
[0052] Embodiment 2, the preparation of vaccine, safety and efficacy test
[0053] 2.1 Preparation of vaccine
[0054] Inoculate the Erysipelothrix suis strain HN1573 into the TSB liquid medium, culture it in a shaker at 37°C, collect the culture after 18 hours, measure the concentration, and adjust the number of bacteria in the culture to 2×10 9 CFU / mL, add 2mL formaldehyde solution per 1000mL bacterial solution, inactivate at 37°C for 12h. Add aluminum hydroxide adjuvant to the inactivated bacterial solution at a volume ratio of 9:1, and mix to prepare the inactivated Erysipelothrix suis inactivated vaccine.
[0055] 2.2 Vaccine Safety Test
[0056] Ten 30-day-old healthy nursery pigs were selected and divided into 2 groups with 5 pigs in each group.
[0057] Vaccine group: inject 4mL of this vaccine subcutaneously in the neck;
[0058] Control group: 4 mL of sterilized normal saline was injected subcutaneously into the neck. The body temperature of the animals was meas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com