Triple vaccine for feline calicivirus infection, feline infectious rhinotracheitis and feline panleukopenia as well as preparation method and application thereof
A technology for leukopenia and feline calicivirus, applied in the field of veterinary biological products, can solve the problems that vaccines cannot protect well, affect vaccine immunity, and low antigen levels, and reduce the number of animal stress , Reduce the number of immunizations, the effect of high antigen level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
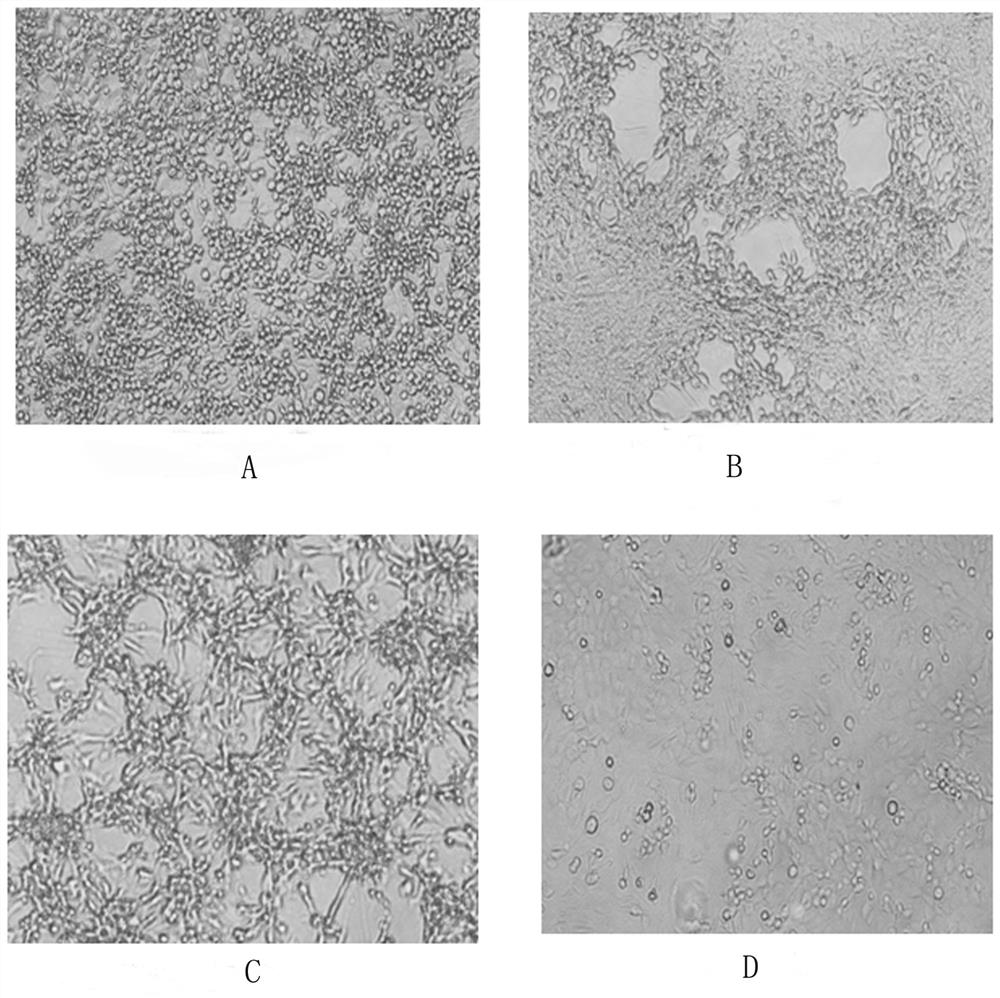
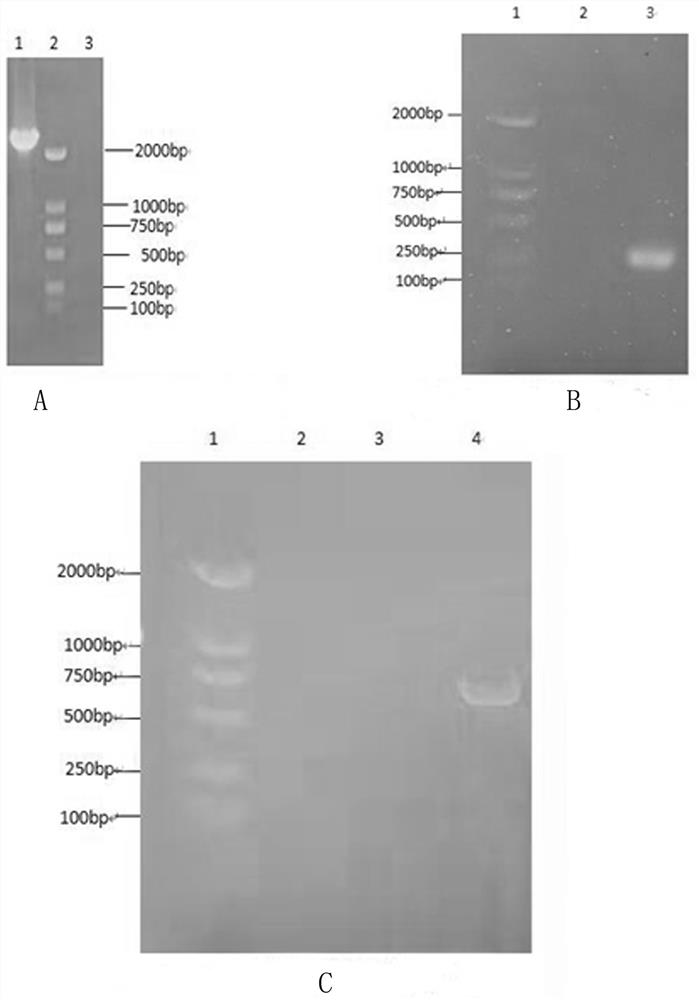
[0032] Embodiment 1: the acquisition of three kinds of strains
[0033] A cat kidney cell line F81S adapted to full suspension culture, named F81S, preserved in the China Center for Type Culture Collection, with the preservation number CCTCC NO: C201799, classified as: cat kidney cell suspension adapted strain F81S, and the preservation date is: On June 29, 2017, the address of the depository unit is: China. Wuhan. Wuhan University.
[0034] Suspension cell growth medium: HYC-RDMP-28 medium from HyClone Company, supplemented with 2% newborn calf serum, the cell growth medium is the nutrient solution used for F81S cell suspension strain culture in shake flasks and bioreactor full suspension culture.
[0035] Adherent cell growth medium: Gibco's DMEM medium, supplemented with 10% newborn bovine serum, the cell growth medium is the nutrient solution used for F81 cell adherent culture.
[0036] Adhesive culture virus culture maintenance medium: DMEM medium of Gibco company, added...
experiment example 2
[0099] Experimental Example 2: Safety Test of Three Kinds of Virus Strains
[0100] Superdose safety test of triple inactivated freeze-dried vaccine for feline calicivirus disease, feline infectious rhinotracheitis and feline panleukopenia of the present invention
[0101] In this experiment, 20 healthy and susceptible kittens aged 2-3 months were selected, and the titers of anti-FCV, anti-FHV and anti-FPV antibodies in cat serum were required to be less than 2. The test was divided into 4 groups, 3 groups in the immunization group, which were inoculated with different batches of vaccines (batch numbers: F-1901, F-1902 and F-1903), with 5 cats in each group and 5 cats in the control group. The immunized group was injected with 2 mL (2 doses) triple inactivated freeze-dried vaccine for feline calicivirus disease, feline infectious rhinotracheitis and feline panleukopenia in the neck. The neck muscles of the control group were inoculated with 2 mL of frozen-thawed F81S cells. ...
experiment example 3
[0106] Experimental Example 3: Efficacy Test of Three Kinds of Vaccines
[0107] Efficacy test of triple inactivated freeze-dried vaccine for feline calicivirus disease, feline infectious rhinotracheitis and feline panleukopenia of the present invention
[0109] In the test, 30 healthy and susceptible kittens aged 2-3 months (required cat serum anti-FCV, anti-FHV and anti-FPV antibody titers are all less than 2), 5 cats in each test group, injected 1.0 mL of vaccine through the neck muscle , each control group had 5 cats, and each neck muscle was inoculated with 1 mL of F81S cell freeze-thaw. 14 days after inoculation, all cats were collected from jugular vein blood, separated serum, tested for feline calicivirus and feline infectious rhinotracheitis virus neutralizing antibody, and measured HI for feline panleukopenia virus. The neutralizing antibody and HI antibody of the cats in the immunized group should not be lower than 1:16, and the neutrali...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com