Method for preparing enoxaparin sodium
A technology of enoxaparin sodium and heparin sodium, which is applied in the field of preparation of enoxaparin sodium, to achieve the advantages of effectiveness and safety, small molecular weight, and long plasma half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
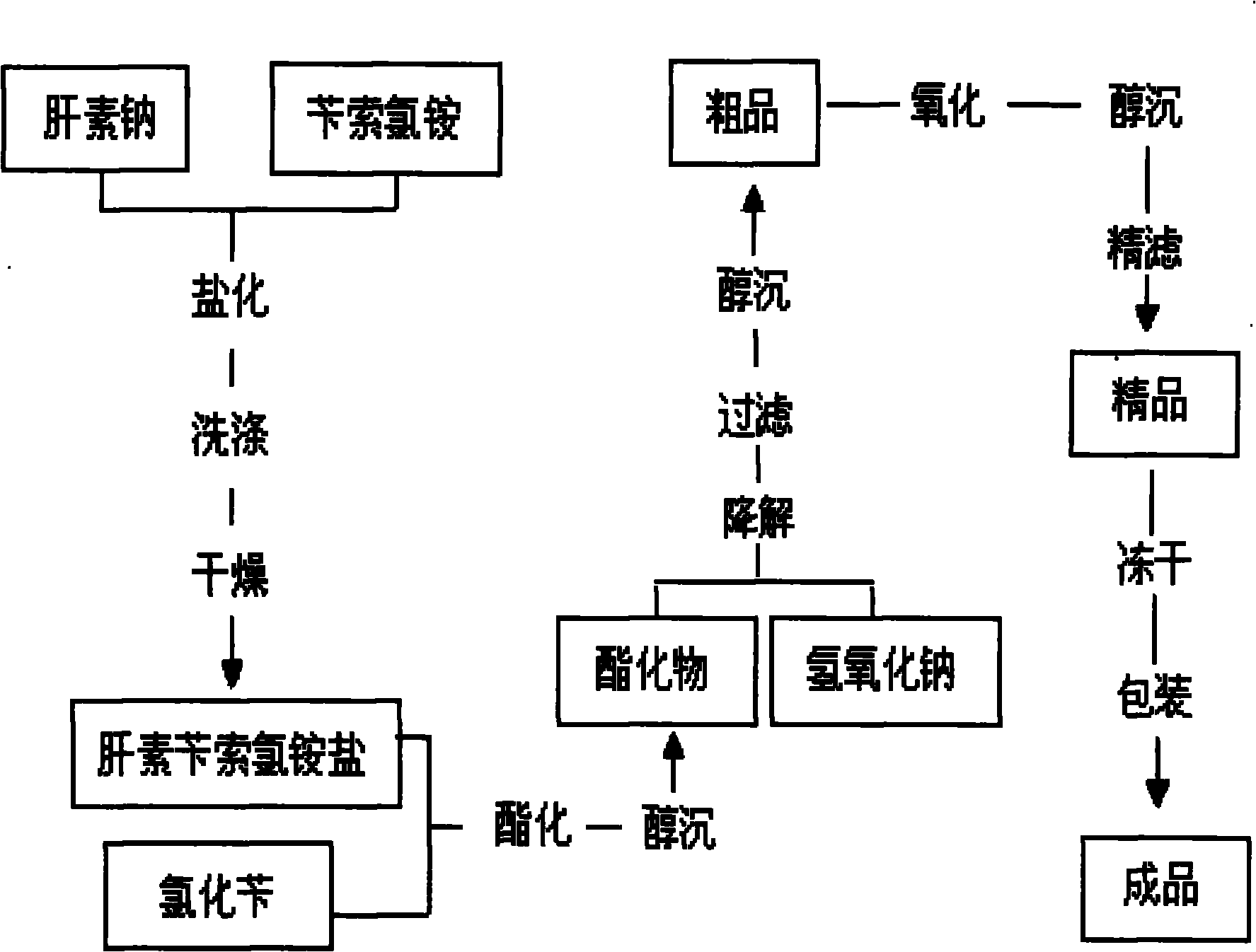
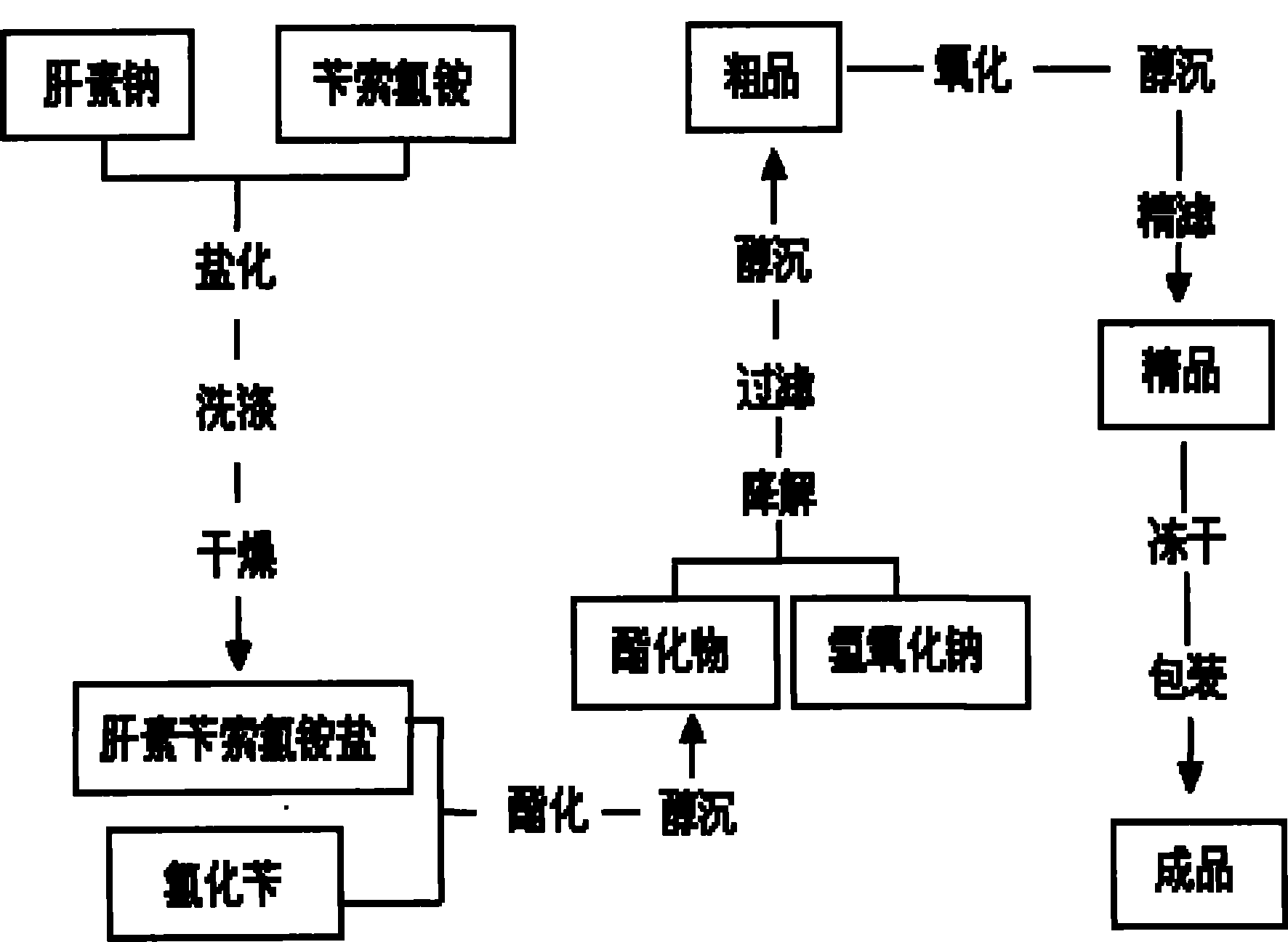
[0023] Embodiment 1: as figure 1 Shown, the present invention prepares enoxaparin sodium technological process as:
[0024] a. take by weighing 3kg heparin sodium (injection grade removes DS), 5kg benzethonium chloride for subsequent use;
[0025] b. Add the weighed heparin sodium into 30 liters of purified water at 30-40°C and stir to dissolve it completely; add the weighed benzethonium chloride into 30 liters of purified water at 45-50°C and stir to dissolve it completely ;
[0026] c. Saltification: Slowly add the above-mentioned dissolved heparin sodium solution into the dissolved benzethonium chloride solution, add while stirring, continue to stir for 30-60 minutes after adding, and then let stand;
[0027] d. Drying: Use a centrifuge to centrifuge the above reactant, discard the supernatant to obtain heparin salt crystals, add 100 liters of purified water to wash the heparin salt crystals, centrifuge after washing, discard the supernatant, repeat the operation 3 to 4 ...
Embodiment 2
[0036] Example 2: The difference between this example and Example 1 is that heparin sodium is 2 kg, benzyl chloride is 6 L, and sodium hydroxide is 165 g.
Embodiment 3
[0037] Example 3: The difference between this example and Example 1 is that the sodium heparin is 4kg, the benzyl chloride is 12L, and the sodium hydroxide is 330g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com