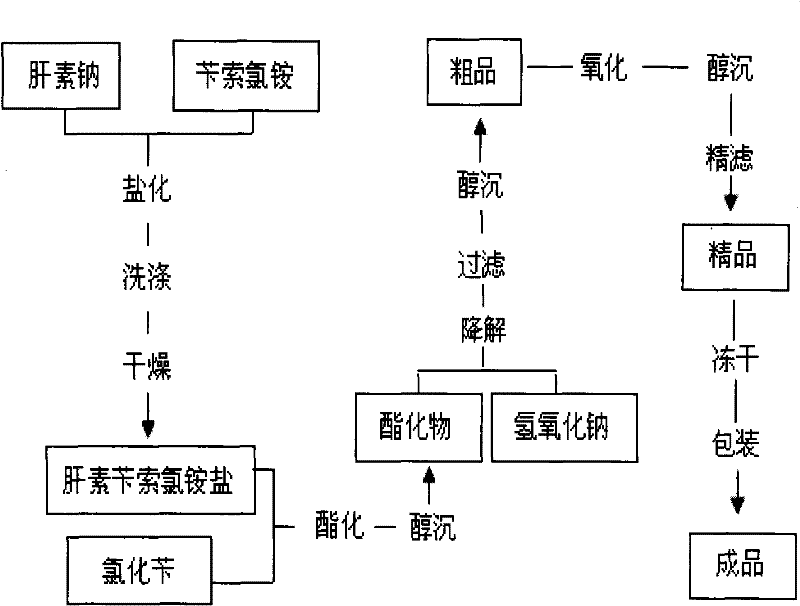
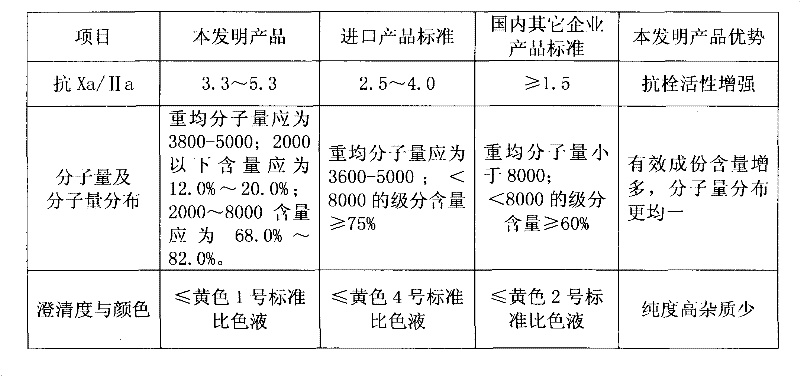
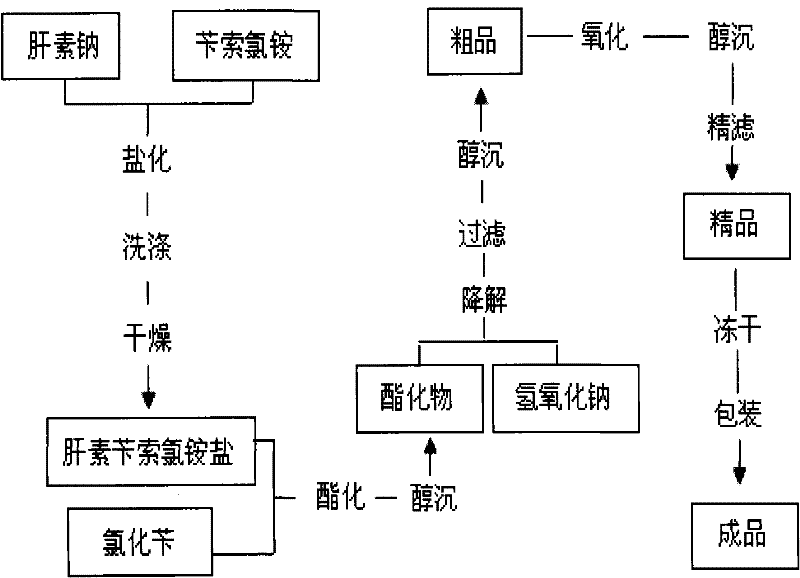
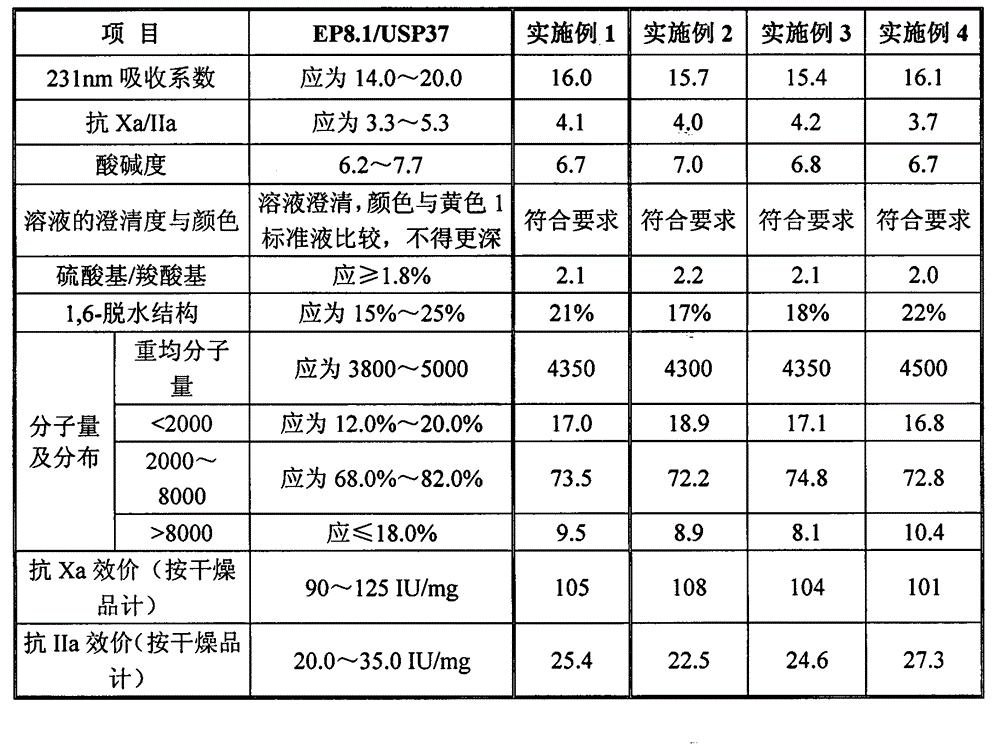
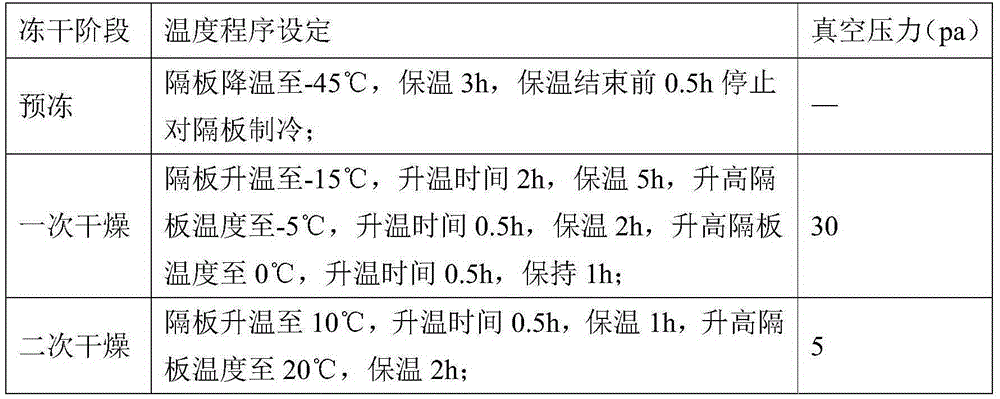
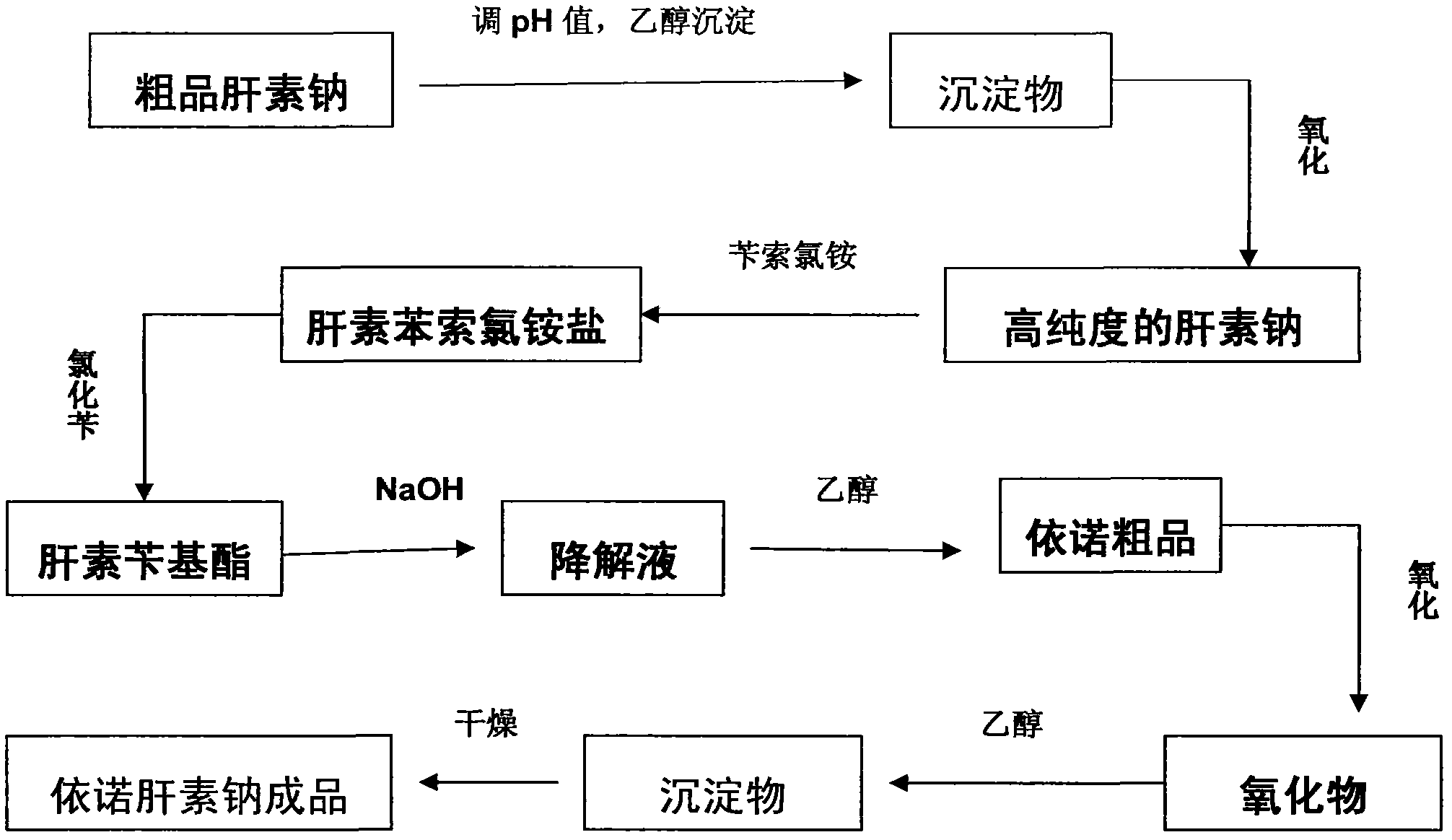
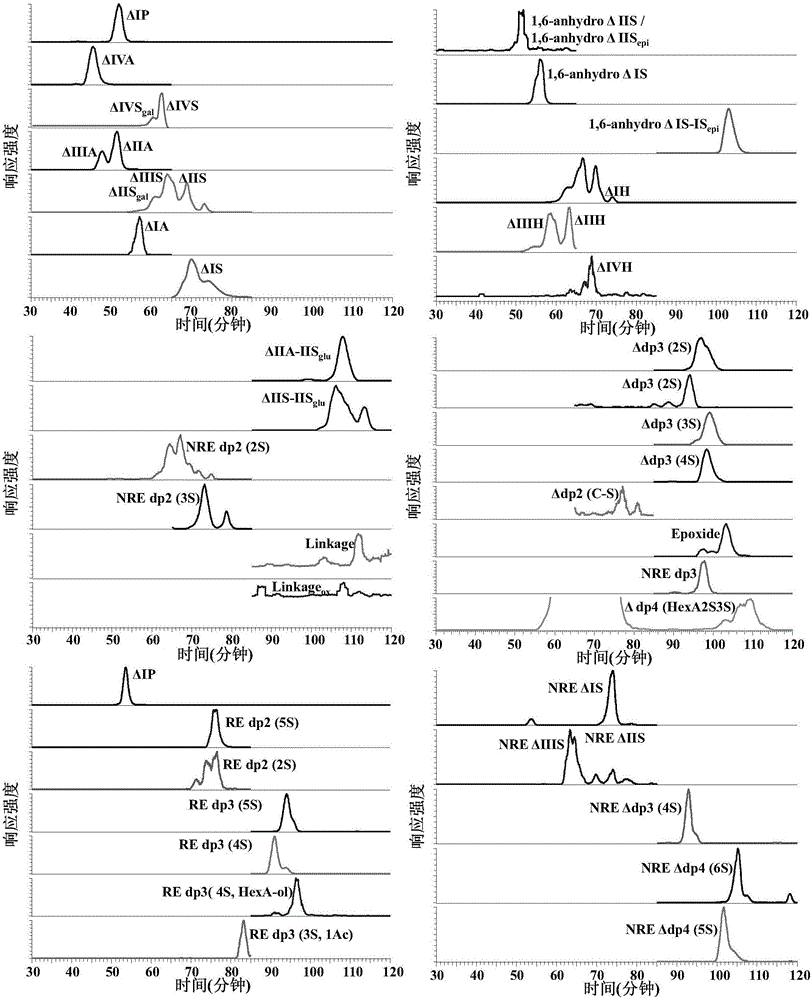
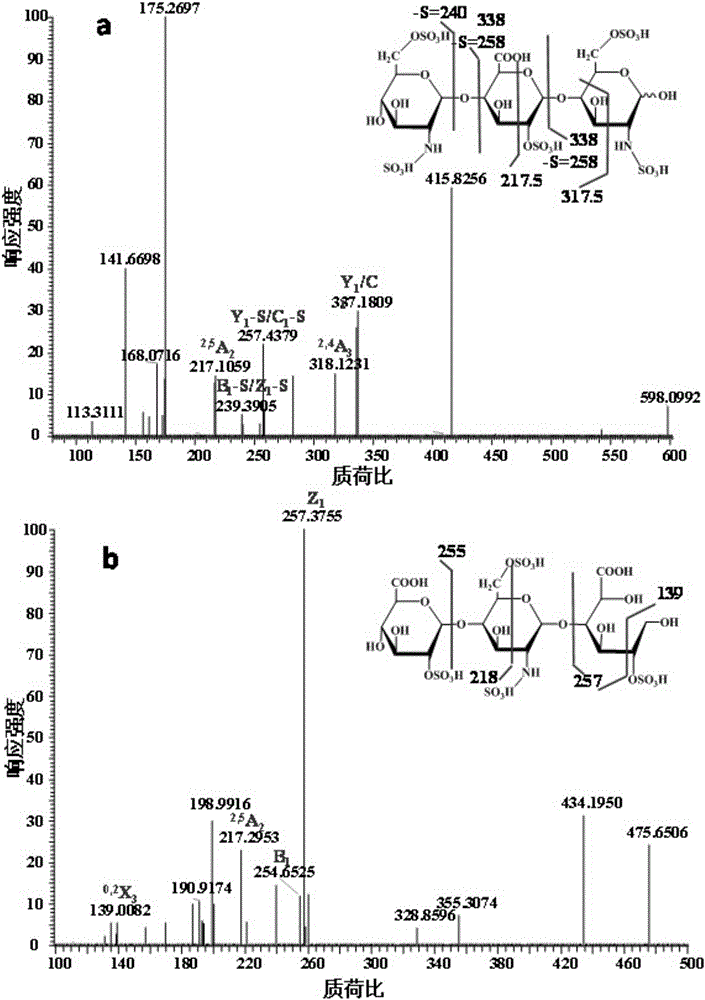
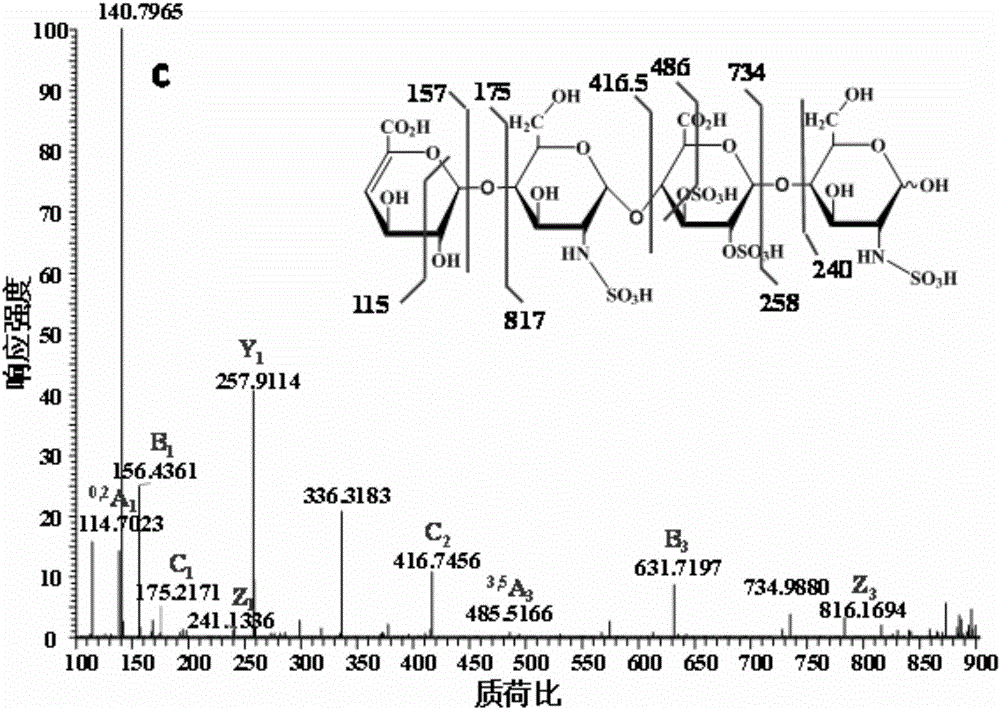
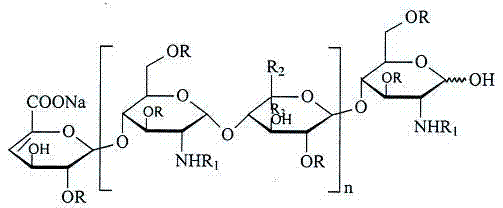
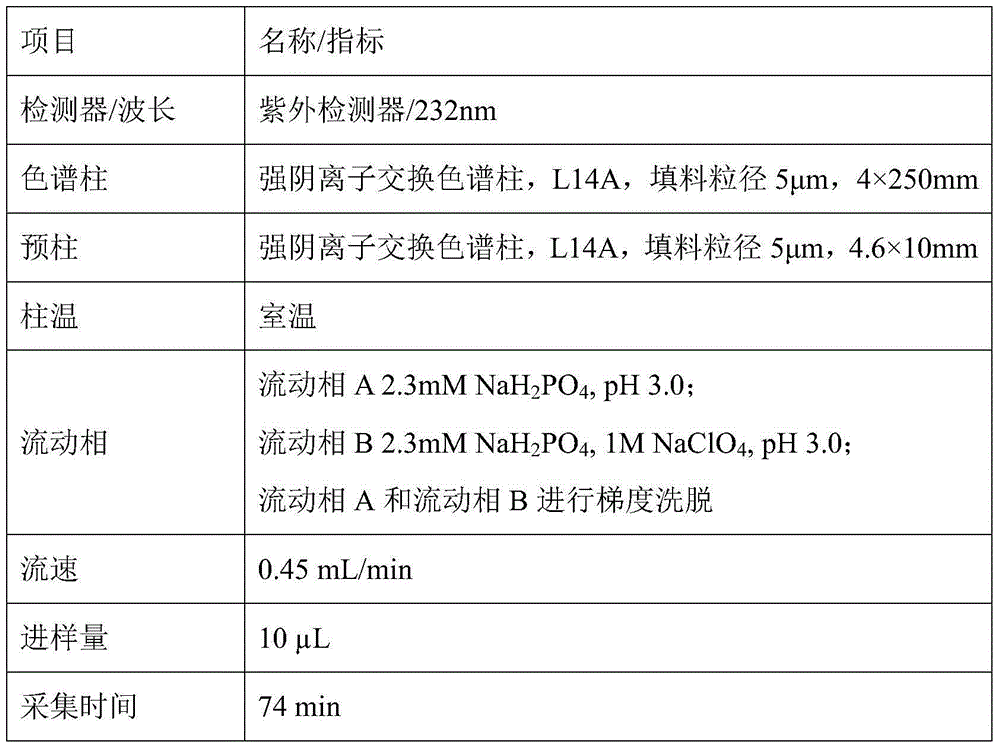
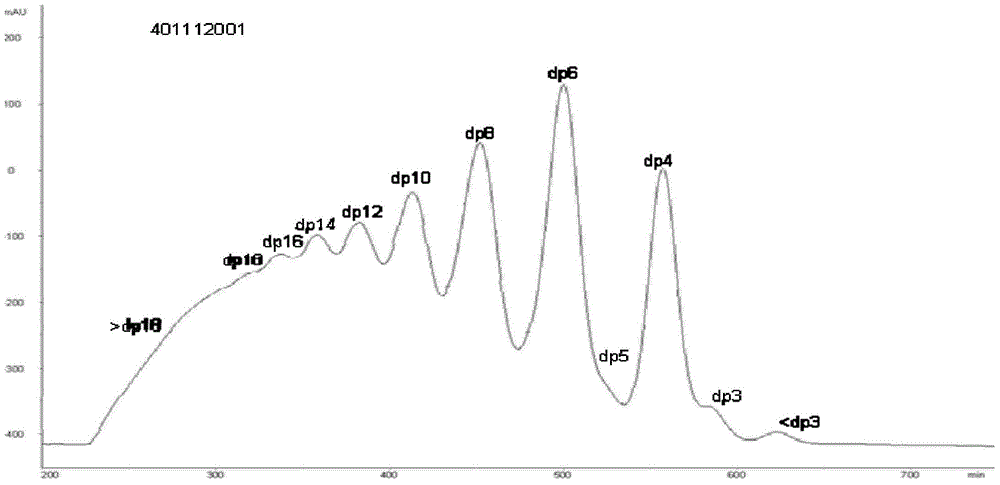
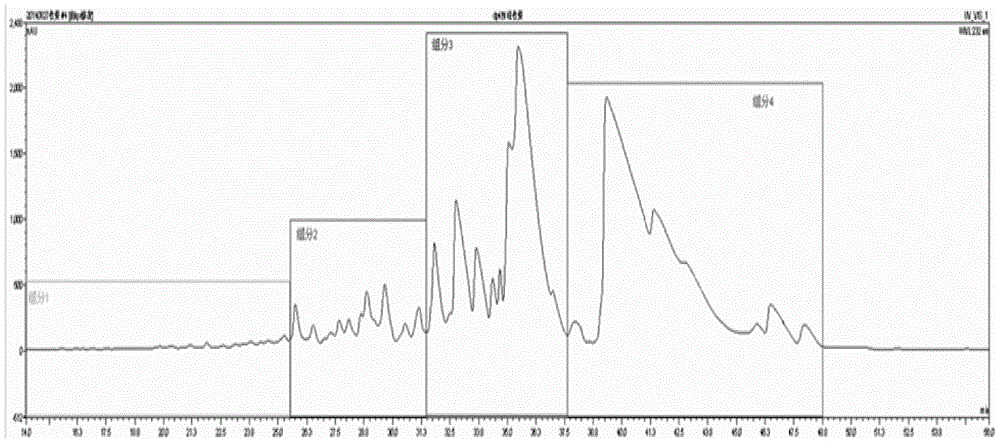
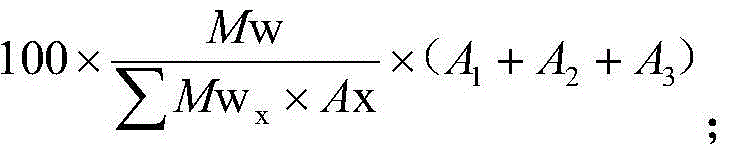Patents
Literature
97 results about "Enoxaparin sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
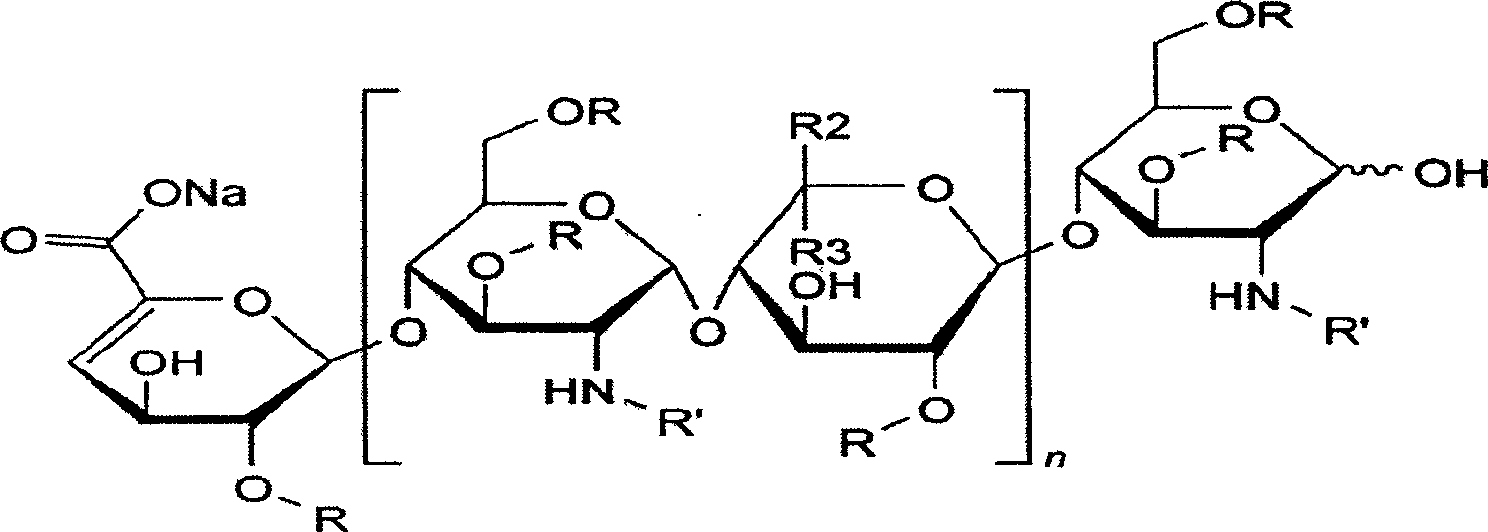
Enoxaparin is used to prevent and treat harmful blood clots.
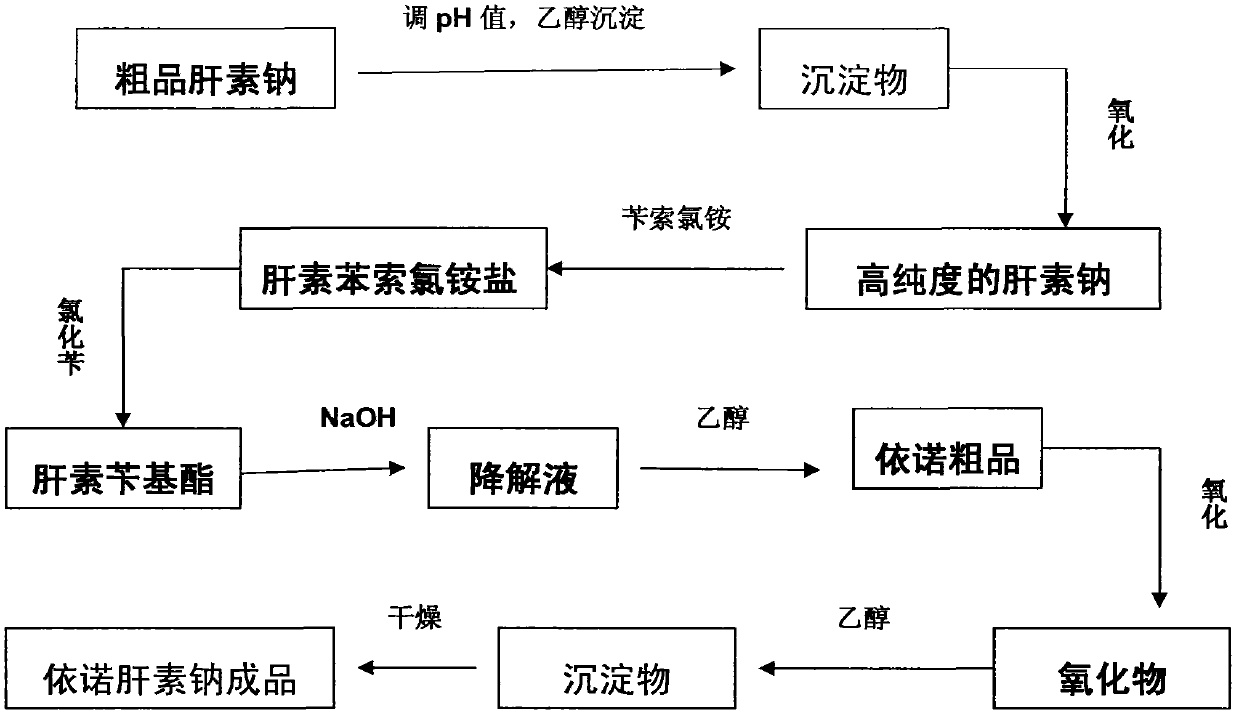
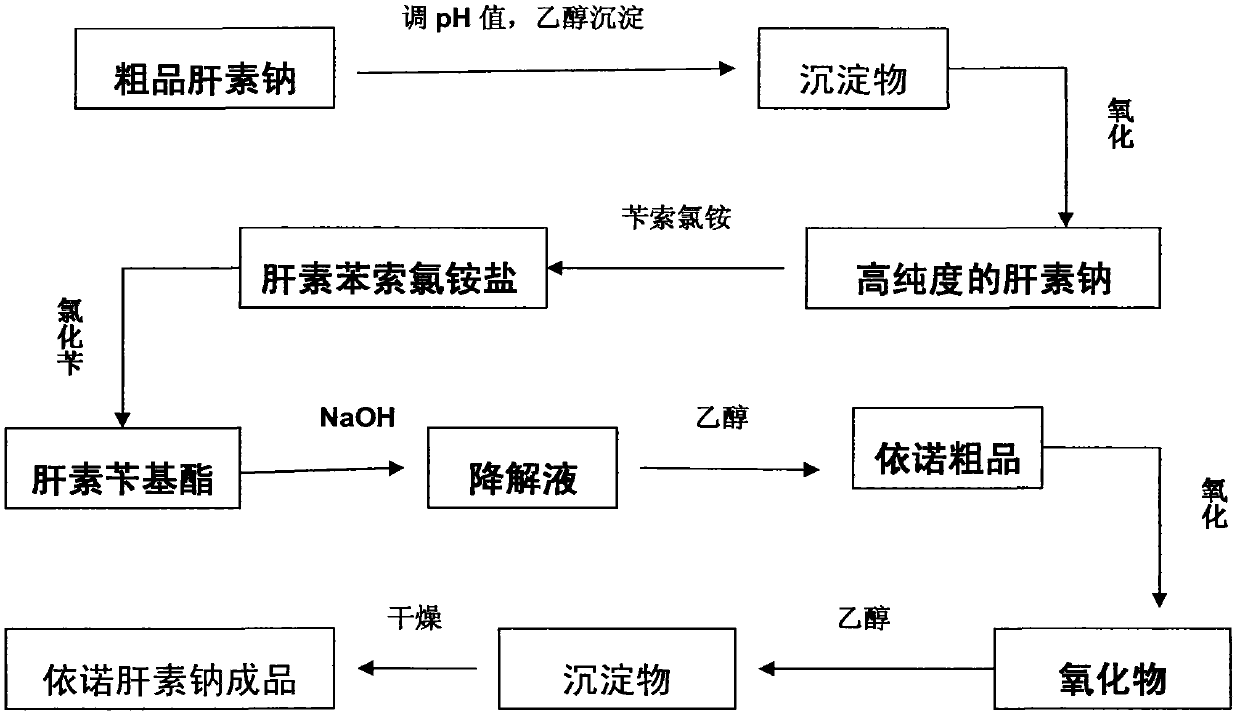
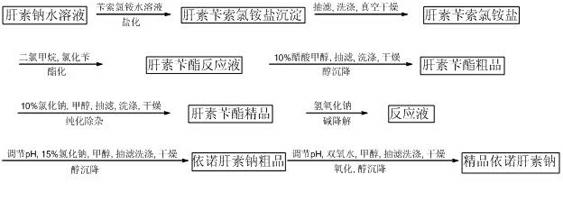
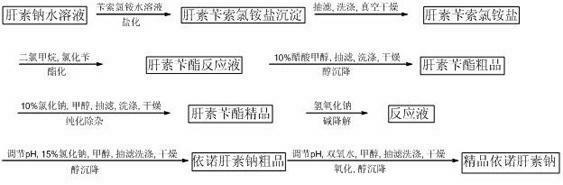
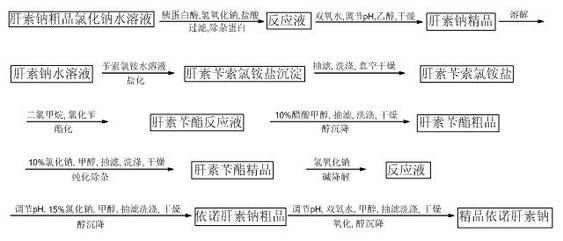
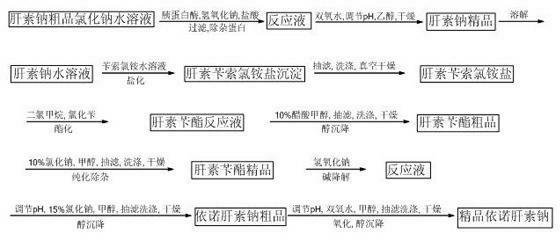
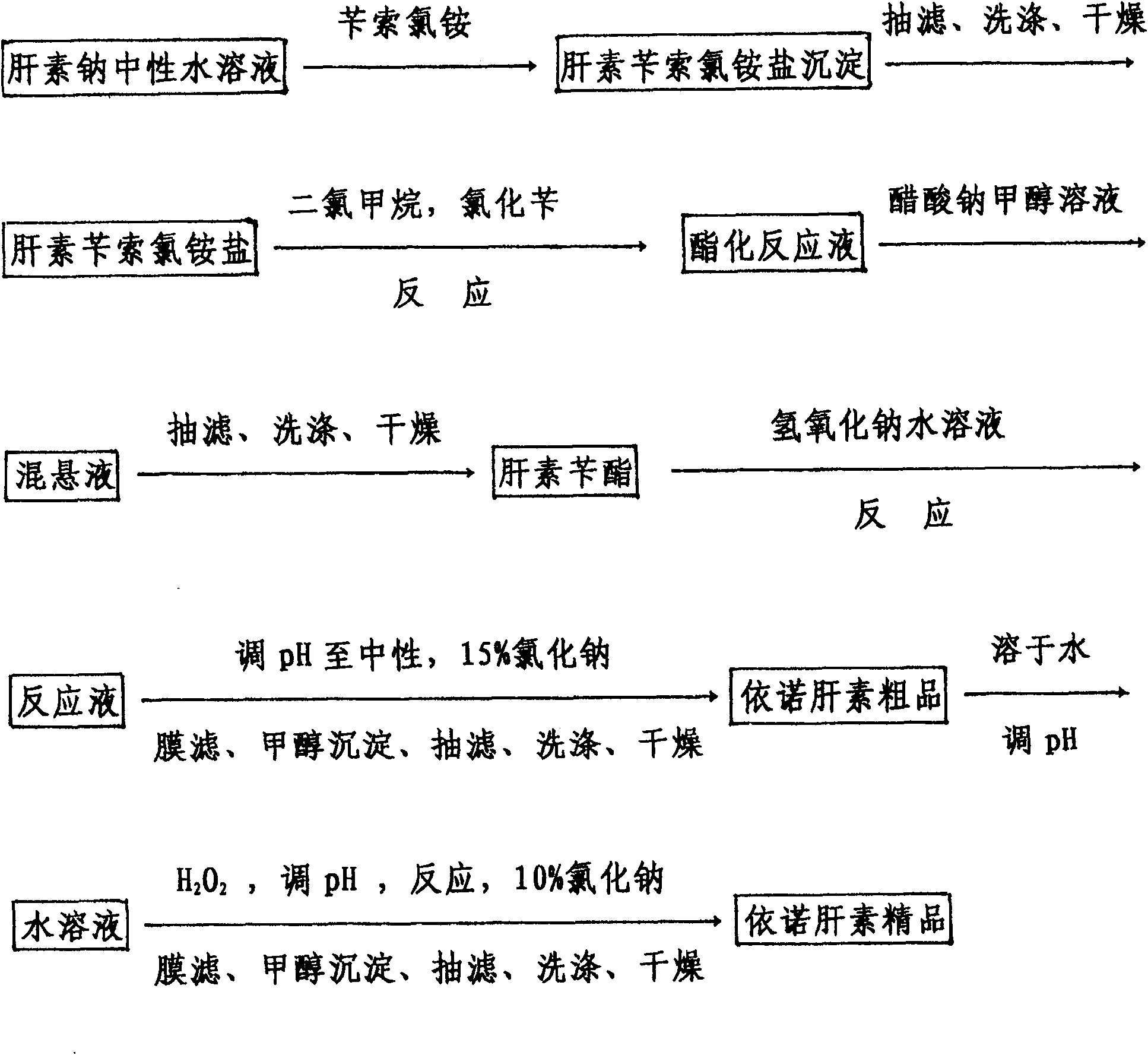
Method for directly producing enoxaparin sodium from crude product heparin sodium
ActiveCN102603925AControl impurity contentReduce intermediate environmentOrganic solventDepolymerization
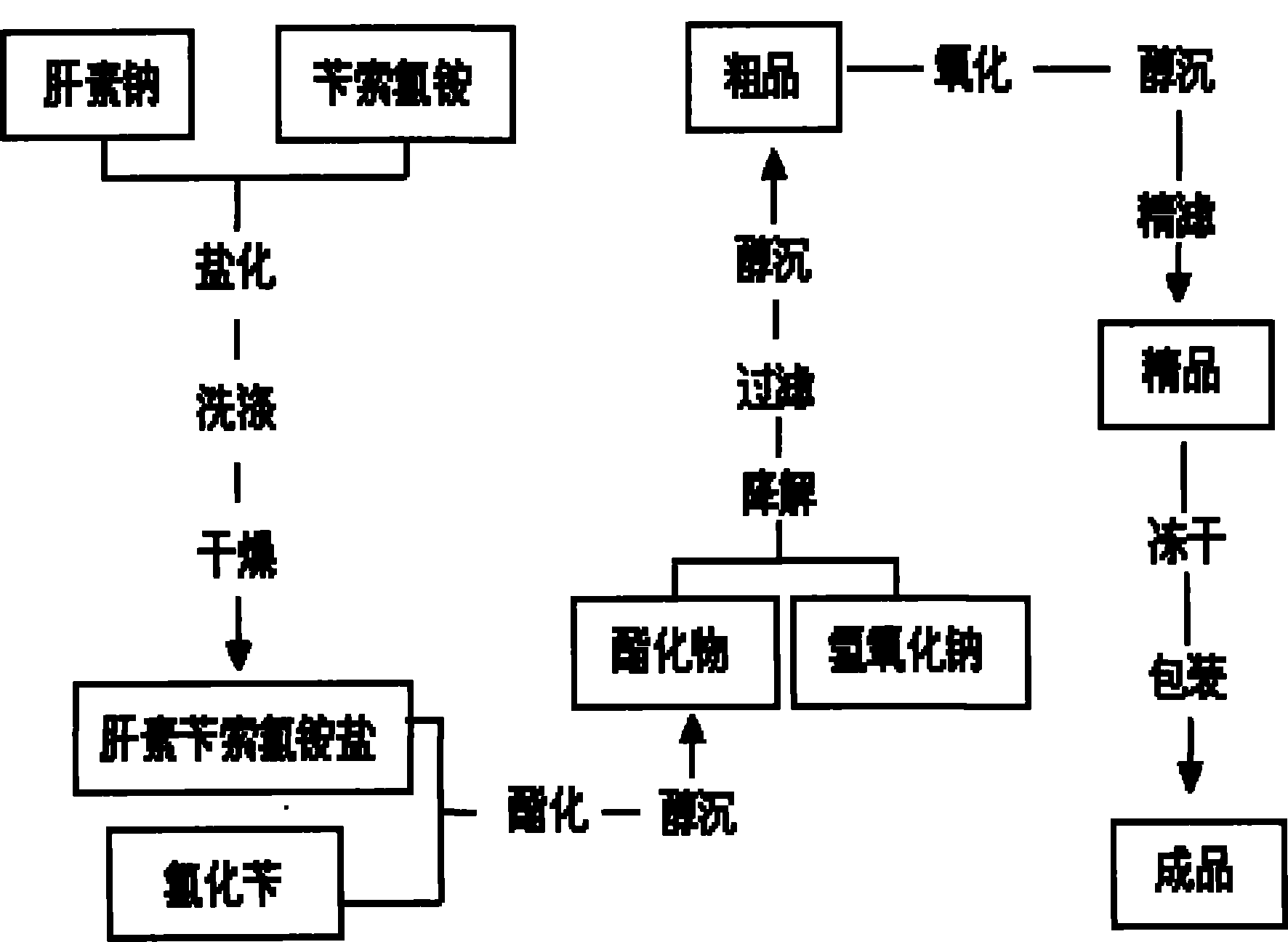
The invention relates to a preparation method for directly producing enoxaparin sodium from crude product heparin sodium. The preparation method comprises the following steps of: taking the crude product heparin sodium as a raw material, performing fractionated precipitation through an organic solvent to remove most of impurities in the crude product heparin sodium, and then removing part of residual impurity proteins, pigments and other impurities by oxidation through hydrogen peroxide so as to get the high-purity heparin sodium which is in line with the production requirements of the enoxaparin sodium; and taking the high-purity heparin sodium as an intermediate product, preparing a heparin quaternary ammonium salt, preparing heparin benzyl ester, performing alkaline depolymerization on the heparin benzyl ester, neutralizing with an acid, performing alcohol precipitation, refining, decoloring, dehydrating and drying to get an enoxaparin sodium finished product. By adopting the method disclosed by the invention, the use of the organic solvent is greatly reduced, the production efficiency is improved, the influences on the environment are reduced, the enoxaparin sodium finished product which achieves or is better than European Pharmacopoeia 7.0 version is obtained, and the method is simple to operate and can realize industrialized production.
Owner:DONGYING TIANDONG PHARM CO LTD
Method for preparing enoxaparin sodium
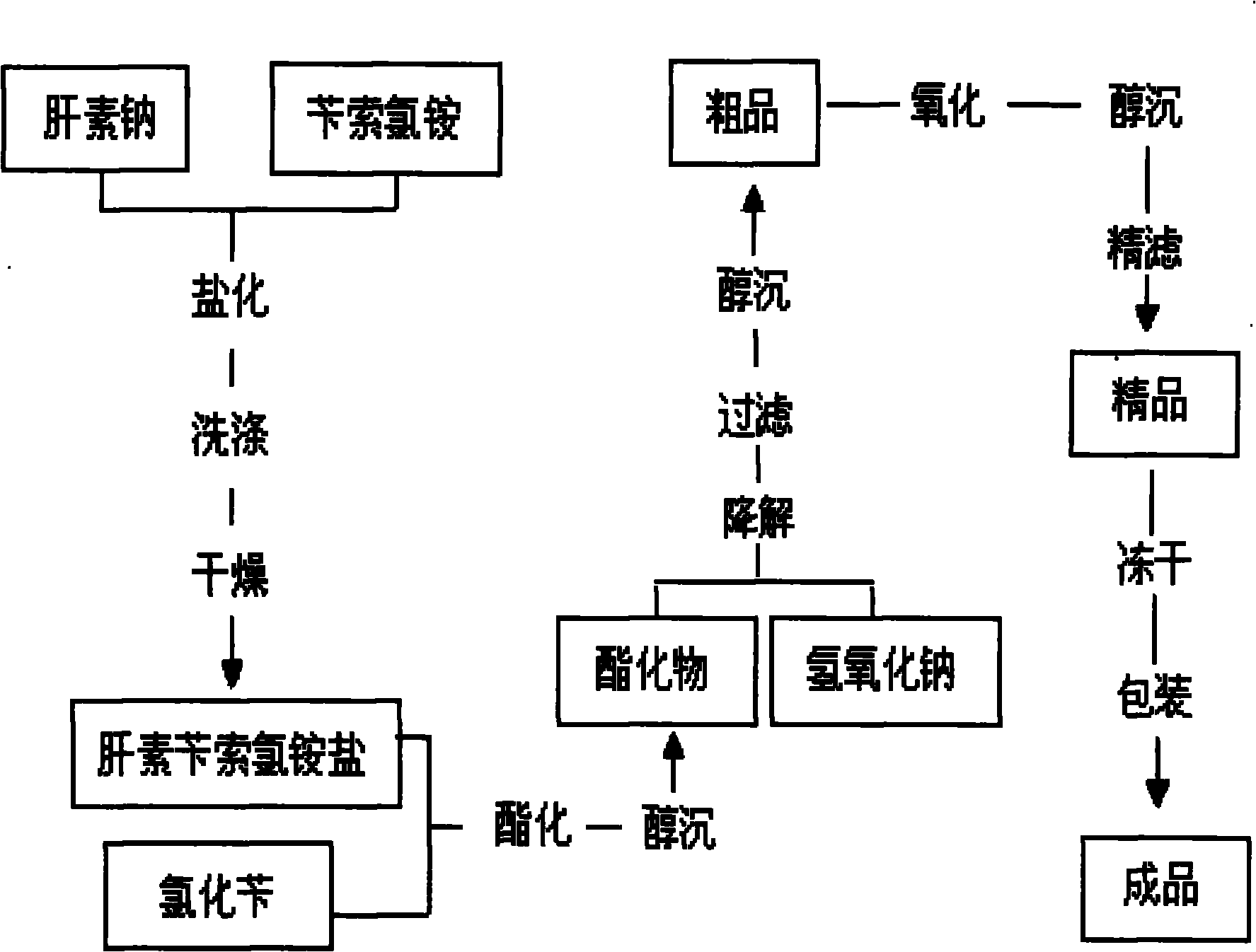
The invention discloses a method for preparing enoxaparin sodium, comprising the steps of salinizing, drying, esterfying, alcohol precipitating, oxidizing, alcohol precipitating, fine filtering and freeze drying. In the method provided by the invention, a hydrophilic liquid phase reaction, a hydrophobic liquid phase reaction and a solid phase reaction are adopted, so that macromolecule sodium heparin is degraded into micromolecule sodium heparin with a specific structure, and the molecular weights of products and molecular weight distribution ranges are controlled, thus anti-FIIa activity resulting in bleeding risk is greatly reduced, the anti-FXa activity is relatively improved, and the product effectiveness and safety advantages are obvious. The enoxaparin sodium can be used for effectively preventing venous thromboembolism and pulmonary embolism, can be used for thrombosis before and after operations of orthopedic surgery and neurosurgery, and can be used for greatly reducing apoplexy risk, more effectively reducing death, cardiac failure and recurrent angina of patients suffering from unstable coronary artery syndromes, reducing hypertriglyceridemia and effectively eliminating the side effects of haemorrhage, osteoporosis and induced thrombocytopenia after long-term use of common unfractionated heparin sodium and derivates of common unfractionated heparin sodium.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Production method for purifying enoxaparin sodium
InactiveCN1850865AWon't breakEfficient separationOther chemical processesBenzyl chlorideSodium heparin
The invention relates to a purifying manufacture method for Yino sodium heparin that adopts long chain quaternary ammonium salt salinization heparin, after taking benzyl chloride esterification, gaining the raw Yino heparin raw product. After taking decoloration by active carbon and macropore adsorptive resin, the sodium heparin product could be gained. The invention is easy to realize industrializing producing.
Owner:HANGZHOU JIUYUAN GENE ENG
Technology for preparing enoxaparin sodium by membrane separation
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Enoxaparin sodium and production purification method thereof
InactiveCN102585037AReduce manufacturing costImprove product qualityBlood disorderExtracellular fluid disorderPurification methodsBenzyl chloride
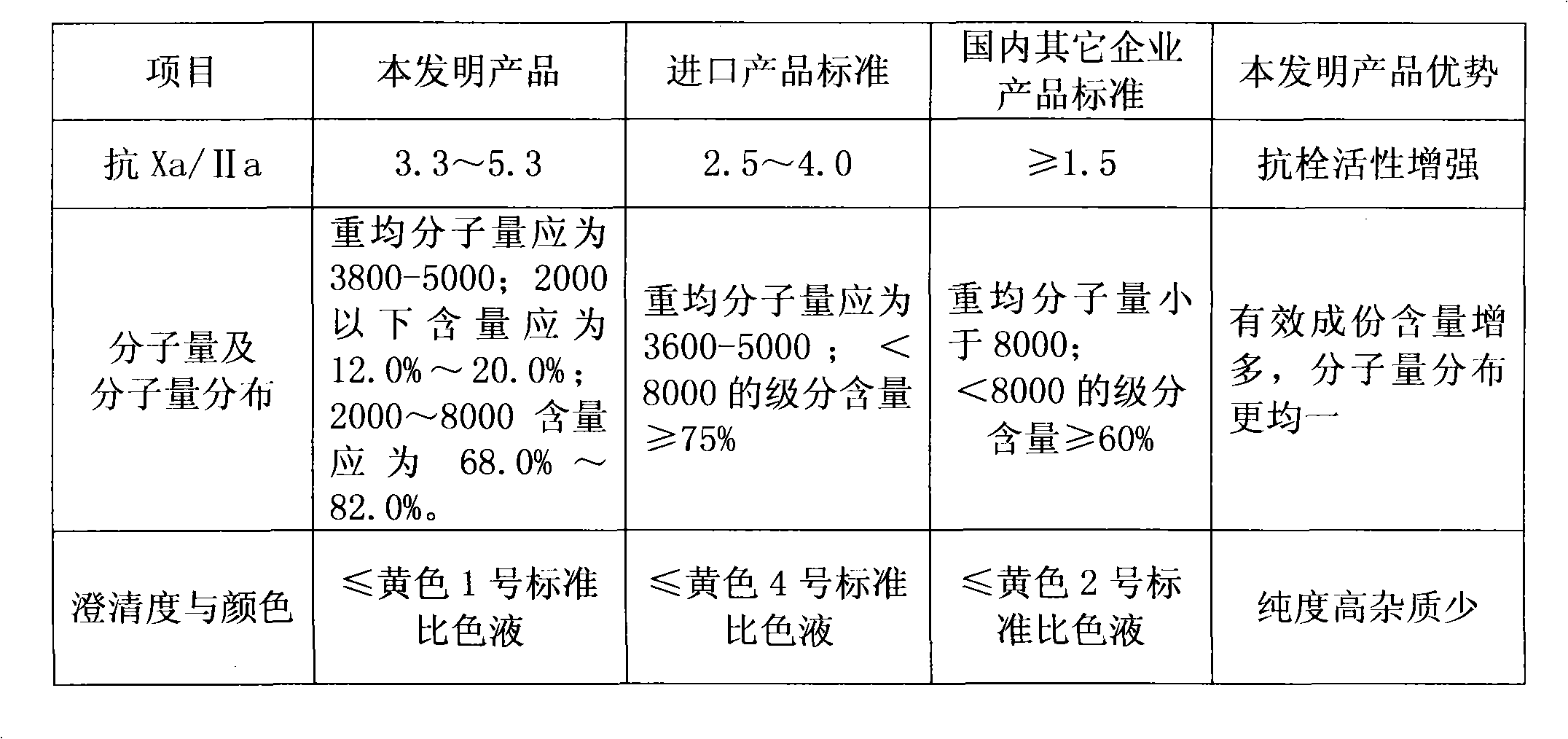
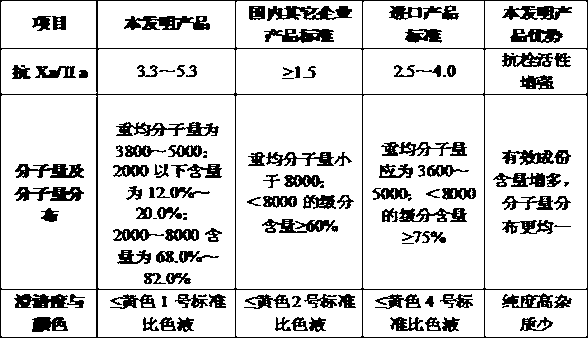
The invention discloses enoxaparin sodium. The average molecular weight of the enoxaparin sodium is between 3500-5500 dalton, thrombolytic biological activity is 100-125IU / mg, and the ratio value of resistance to Xa and IIa resistance is between 3.3-5.3. A production purification method of the enoxaparin sodium comprises preparation of heparin benzyl chloride ammonium salt, preparation of heparin benzyl ester, purification of heparin benzyl ester, preparation of enoxaparin sodium and purification of enoxaparin sodium. By means of the enoxaparin sodium, the molecular weight and distribution range of a product are controlled, and the quality of a fine enoxaparin sodium product meets the quality standard of the european pharmacopoeia. The enoxaparin sodium adopts a crude product of heparin sodium as an initial raw material and can effectively reduce production cost. The refined heparin benzyl ester stabilizes the final quality of the product. Purification difficulties are simplified, the coloring problem in the production is effectively solved, and the product quality is improved.
Owner:麦科罗夫(南通)生物制药有限公司
Production method for purifying enoxaparin sodium
InactiveCN100436483CWon't breakEfficient separationOther chemical processesBenzyl chlorideSodium heparin
The invention relates to a purifying manufacture method for Yino sodium heparin that adopts long chain quaternary ammonium salt salinization heparin, after taking benzyl chloride esterification, gaining the raw Yino heparin raw product. After taking decoloration by active carbon and macropore adsorptive resin, the sodium heparin product could be gained. The invention is easy to realize industrializing producing.
Owner:HANGZHOU JIUYUAN GENE ENG
Decoloration method of enoxaparin sodium
ActiveCN102757516ALow impurity contentImprove securityIon-exchange process apparatusIon-exchanger regenerationHigh concentrationDesalination
The invention discloses a decoloration method of enoxaparin sodium. The method includes dissolving an intermediate of the enoxaparin sodium to be discolored into an ammonium sulfate solution to obtain an enoxaparin sodium solution; when a hydrophobic chromatographic column is balanced by the ammonium sulfate solution, loading the enoxaparin sodium solution to the hydrophobic chromatographic column, and collecting penetration solution; when an anion exchange chromatographic column is balanced by purified water, diluting the penetration solution with the purified water, loading the penetration fluid to the anion exchange chromatographic column, washing the column with the purified water and a sodium chloride solution with a low concentration sequentially after the loading, eluting the column with a sodium chloride solution with a high concentration, and collecting the eluent; subjecting the eluent to nanofiltration, desalination and concentration until the concentration of the enoxaparin sodium in the concentrated solution is 5wt%-20wt%; and precipitating and drying the concentrated solution to obtain the finished product of the enoxaparin sodium. According to the decoloration method, the decoloration effect is good, the color of the finished product is lighter than that of a color solution BY6, the finished product conforms to the provision of European Pharmacopoeia 7.0, and the method is high in safety and easy to control.
Owner:CHANGZHOU QIANHONG BIOPHARMA
Preparation method for low-molecular heparin originated from new species
The invention relates to a preparation method for low-molecular heparin originated from a new species. The preparation method comprises the following steps: (1) fully dissolving heparin sodium prepared from raw materials bovine lungs, ox intestines and / or goat intestines in water, and adding a benzethonium chloride solution to prepare heparin benzethonium chloride salt; (2) dissolving the heparin benzethonium chloride salt in dichloromethane, adding benzyl chloride, stirring the mixture to react, reducing the temperature and adding a sodium acetate methanol solution to prepare heparin benzyl ester; (3) dissolving the heparin benzyl ester in water, adding sodium hydroxide, reducing the temperature after reaction, adjusting the pH to neutral, adding sodium chloride, and then adding alcohol to prepare an enoxaparin sodium crude product; and (4) dissolving the enoxaparin sodium coarse product in water, and purifying and drying the mixture to obtain enoxaparin sodium. According to the preparation method provided by the invention, the condition that the low-molecular heparin can be obtained by heparin sodium prepared from bovine lungs, ox intestines and goat intestines is found for the first time, and the prepared low-molecular heparin is lower in molecular weight compared with that of existing low-molecular heparin, so that the biological activity is higher and the low-molecular heparin has a wide market application prospect.
Owner:SHANDONG UNIV
Method for preparing enoxaparin sodium
The invention discloses a method for preparing enoxaparin sodium, comprising the steps of salinizing, drying, esterfying, alcohol precipitating, oxidizing, alcohol precipitating, fine filtering and freeze drying. In the method provided by the invention, a hydrophilic liquid phase reaction, a hydrophobic liquid phase reaction and a solid phase reaction are adopted, so that macromolecule sodium heparin is degraded into micromolecule sodium heparin with a specific structure, and the molecular weights of products and molecular weight distribution ranges are controlled, thus anti-FIIa activity resulting in bleeding risk is greatly reduced, the anti-FXa activity is relatively improved, and the product effectiveness and safety advantages are obvious. The enoxaparin sodium can be used for effectively preventing venous thromboembolism and pulmonary embolism, can be used for thrombosis before and after operations of orthopedic surgery and neurosurgery, and can be used for greatly reducing apoplexy risk, more effectively reducing death, cardiac failure and recurrent angina of patients suffering from unstable coronary artery syndromes, reducing hypertriglyceridemia and effectively eliminatingthe side effects of haemorrhage, osteoporosis and induced thrombocytopenia after long-term use of common unfractionated heparin sodium and derivates of common unfractionated heparin sodium.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Method for producing enoxaparin sodium by using crude sodium heparin products
ActiveCN104558252AGuarantee product qualityFree from multiple oxidationDepolymerizationFreeze-drying
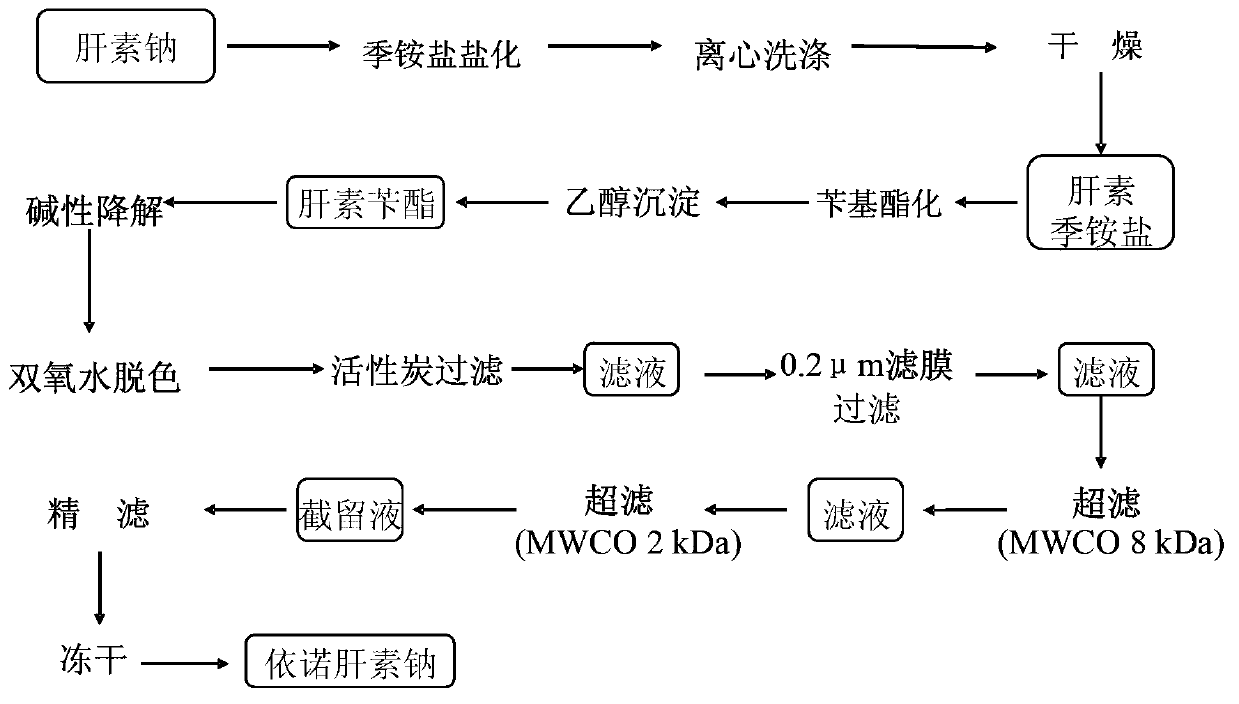
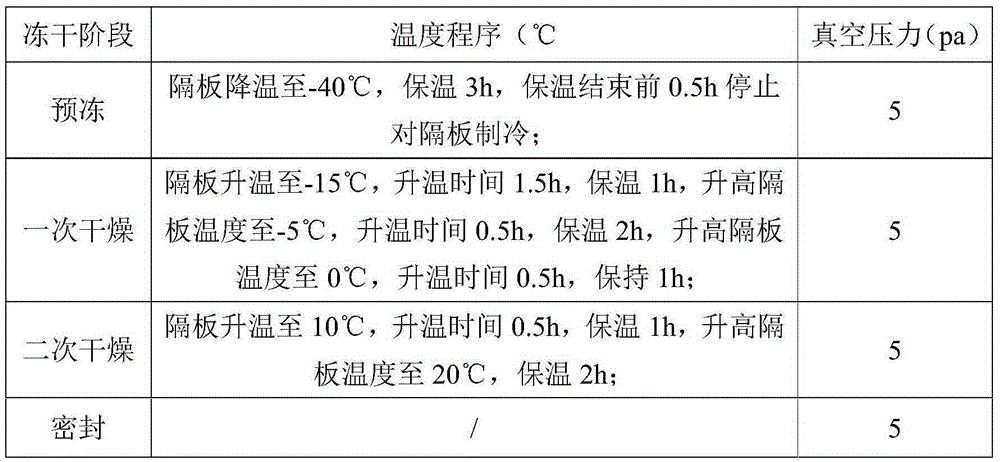
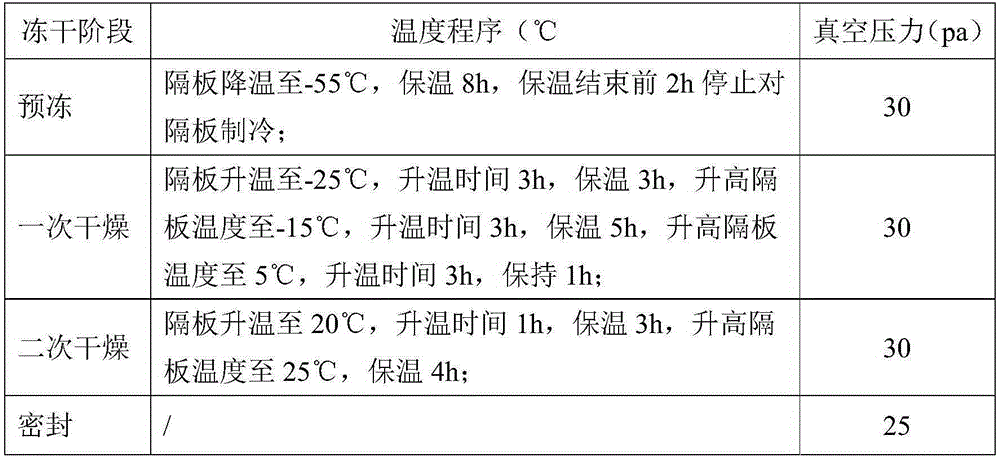
The invention discloses a method for producing enoxaparin sodium by using crude sodium heparin products. The method is implemented by taking crude sodium heparin products as a raw material through the steps of pretreating the crude sodium heparin products by using a salt hydrolysis process; sequentially carrying out oxidation, ion exchange resin adsorption, washing and elution on the obtained object; sequentially carrying out ultrafiltration and freeze-drying on the obtained product so as to obtain a fine sodium heparin product; and sequentially carrying out salifying, esterification, depolymerization, oxidation, alcoholic precipitation, and freeze-drying treatment on the fine sodium heparin product, so that enoxaparin sodium is obtained. The method disclosed by the invention has the advantages that the quality of enoxaparin sodium is controlled from the aspects of source and process, the production cycle is short, the energy consumption of production is low, and the quality of products is high, therefore, the method is suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HUAKUN HEBEI BIOTECH
Islamic enoxaparin sodium and method for producing and purifying same
InactiveCN102585038ATo satisfy the market's needsReduce manufacturing costBlood disorderExtracellular fluid disorderAlcoholHeparin sodium
The invention discloses islamic enoxaparin sodium. The average molecular weight of the islamic enoxaparin sodium is 3,500 to 5,500 Daltons, thrombolytic bioactivity is 100 to 125IU / mg, and the ratio of Xa resistance to IIa resistance is 3.3 to 5.3. A method for producing and purifying the islamic enoxaparin sodium comprises the following steps of: purifying a crude heparin sodium product, salinizing heparin sodium, drying, esterifying, performing alcohol precipitation, performing alkali degradation, performing alcohol precipitation, oxidizing, performing alcohol precipitation, performing fine filtering, and drying to obtain a finished product. The crude bovine lung heparin sodium product is taken as an initial raw material, so that production cost can be effectively produced; the heparin sodium is purified by trypsin, so that the method is convenient to operate, yield is high, and the prepared fine heparin sodium product has the advantages of stable quality, high purity, high titer and the like; and by refining heparin benzyl ester, the quality of a final product is stabilized, purification difficulty is reduced, the problem that a pigment is generated during production is effectively solved, and the quality of the product is improved.
Owner:麦科罗夫(南通)生物制药有限公司
Clexane and preparation method thereof
ActiveCN100582123CReduce manufacturing costImprove product qualityOrganic active ingredientsBlood disorderSodium acetateBenzyl chloride
The present invention is enoxaparin and its preparation process, and features that the preparation process includes the following steps: dissolving sodium heparin in water, dissolving benzethonium chloride in water, mixing these two kinds of solution to react, vacuum suction filtering, washing the solid with water and stoving to obtain quaternary ammonium salt of heparin; dissolving the salt in dichloromethane, adding benzyl chloride to react, adding methanol solution of sodium acetate to produce precipitate, suction filtering, washing the solid with methanol and stoving to obtain heparin benzyl ester; dissolving heparin benzyl ester in 0.1N water solution of sodium hydroxide, adding sodium chloride, membrane filtering after dissolving, adding methanol to separate out precipitate, suction filtering, washing and stoving solid to obtain coarse enoxaparin sodium product; and purifying to obtain refined enoxaparin sodium product. The present invention has low production cost and high product quality.
Owner:JIANGSU ALAND NOURISHMENT
Enoxaparin sodium injection and preparation process thereof
ActiveCN104013570AImprove stabilityDrop in color darkenOrganic active ingredientsPharmaceutical delivery mechanismVitamin CMedicine
The invention belongs to the pharmaceutic preparation field, and in particular relates to an enoxaparin sodium injection and a preparation process thereof. The processes of adding antioxygen of the assigned type and regulating the pH are applied to the preparation of the enoxaparin sodium injection. The injection is specifically prepared from enoxaparin sodium, vitamin C, L-cysteine and water for injection, the pH regulating agent is used for regulating the pH of the injection at 5.5-7.5. Compared with the prior art, the technical scheme provided by the invention is capable of greatly improving the stability of the enoxaparin sodium injection, the aspects that the color of the medicine liquor is darkened, the activity is reduced, and the pH of the medicine liquor is greatly reduced are obviously improved; and the process is simple and suitable for the large-scale industrial production.
Owner:SHANDONG NEWTIME PHARMA
Sheep enoxaparin sodium compound preparation method, compound and application of compound
InactiveCN105131153AQuality is easy to controlRaw materials are easy to getOrganic active ingredientsPharmaceutical delivery mechanismSheep farmingMedicine
The invention discloses a method for preparing sheep enoxaparin sodium from sheep intestinal mucosa heparin. The method comprises the following steps: 1, preprocessing sheep heparins; 2, preparing a sheep heparin quaternary ammonium salt; 3, preparing sheep heparin benzyl ester; and 4, carrying out alkali depolymerization on the sheep heparin benzyl ester, decoloring, neutralizing by using an acid, carrying out alcohol precipitation, refining, and drying to obtain finished sheep enoxaparin sodium. The simple and efficient method for preparing the sheep enoxaparin sodium from the sheep intestinal mucosa heparin is screened and established, and researches of the systemic physical and chemical properties, the biological activity and the molecule structure are carried out on the prepared sheep enoxaparin sodium. The sheep enoxaparin sodium prepared in the invention completely accords with USP37 and EP8.0 quality release criteria of sheep enoxaparin sodium, and has extremely high practical values and medical application prospect. The sheep enoxaparin sodium has the advantages of simple and easily available raw material, controllable quality, no existence of bovine spongiform encephalopathy virus risk, promotion of the effective utilization of sheep culture and slaughter wastes (intestinal mucosa), and great economy potential.
Owner:SUZHOU RONGXI BIOTECH CO LTD
Decoloration method for enoxaparin sodium intermediate
ActiveCN103214597AMild conditions for oxidative decolorizationStructural damage is smallHigh concentrationDissolution
The invention discloses a decoloration method for an enoxaparin sodium intermediate. The decoloration method for the enoxaparin sodium intermediate comprises the following operation steps of: (1) dissolving the enoxaparin sodium intermediate by sodium chloride solution to obtain dissolution solution; (2) adding the dissolution solution obtained in the step (1) in hydrogen peroxide solution, and filtering to obtain filtrate; (3) adsorbing the filtrate obtained in the step (2) by macroporous anion exchange resin, sequentially washing by purified water and low-concentration sodium chloride solution, and eluting by high-concentration sodium chloride solution to obtain eluate after the adsorption is complete; and (4) precipitating the eluate obtained in the step (3) by ethanol, dehydrating, and vacuum-drying to obtain the enoxaparin sodium finished product.
Owner:山东万邦赛诺康生化制药股份有限公司
Production method for enoxaparin sodium
The invention relates to a production method for enoxaparin sodium. Started from an original material (macromolecule heparin sodium), the technical processing under a specific condition is performed and a suitable solvent is selected, an end product of enoxaparin sodium meeting European pharmacopoeia standard can be acquired, the environmental pollution is less, the yield is high and the molecular weight and titer of the finally acquired end product can be efficiently and perfectly controlled.
Owner:SUZHOU ERYE PHARMA CO LTD
Production method of enoxaparin sodium
The invention discloses a production method of enoxaparin sodium, which comprises the following steps: carrying out enzymolysis on heparin sodium, carrying out HPLC (high performance liquid chromatography) analysis, calculating the contents of various peaks by a peak area normalization process, determining the varieties of disaccharides and tetrasaccharides of the heparin sodium subjected to enzymolysis according to a standard spectrum of disaccharides and tetrasaccharides after heparin sodium enzymolysis, comparing with the contents of disaccharides and tetrasaccharides of the standard spectrum, and selecting the satisfactory heparin sodium raw material to produce the enoxaparin sodium. The method is simple and effective, can ensure the safety and effectiveness of the product, controls the quality of the enoxaparin sodium from the source, can avoid the waste of the active pharmaceutical ingredient heparin sodium, greatly saves the cost and enhances the production efficiency.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Enoxaparin injection preparation and industrial production method thereof
ActiveCN105362238AImprove stabilityHigh clinical safetyOrganic active ingredientsPowder deliveryOrganic chemistryEnoxaparin sodium
The invention provides an enoxaparin injection preparation. The enoxaparin injection preparation is prepared from, by weight, 20 parts of enoxaparin sodium and 1-2 parts of stent agent. The invention further provides an industrial production method of the enoxaparin sodium injection preparation. The enoxaparin injection preparation is good in stability and clinical safety, simple in preparation technology, appropriate in cost and suitable for mass industrial production.
Owner:CHENGDU BAIYU JINGELAI PHARMA CO LTD
Method for directly producing enoxaparin sodium from crude product heparin sodium
ActiveCN102603925BControl impurity contentReduce intermediate environmentDepolymerizationOrganic solvent
The invention relates to a preparation method for directly producing enoxaparin sodium from crude product heparin sodium. The preparation method comprises the following steps of: taking the crude product heparin sodium as a raw material, performing fractionated precipitation through an organic solvent to remove most of impurities in the crude product heparin sodium, and then removing part of residual impurity proteins, pigments and other impurities by oxidation through hydrogen peroxide so as to get the high-purity heparin sodium which is in line with the production requirements of the enoxaparin sodium; and taking the high-purity heparin sodium as an intermediate product, preparing a heparin quaternary ammonium salt, preparing heparin benzyl ester, performing alkaline depolymerization on the heparin benzyl ester, neutralizing with an acid, performing alcohol precipitation, refining, decoloring, dehydrating and drying to get an enoxaparin sodium finished product. By adopting the method disclosed by the invention, the use of the organic solvent is greatly reduced, the production efficiency is improved, the influences on the environment are reduced, the enoxaparin sodium finished product which achieves or is better than European Pharmacopoeia 7.0 version is obtained, and the method is simple to operate and can realize industrialized production.
Owner:DONGYING TIANDONG PHARM CO LTD
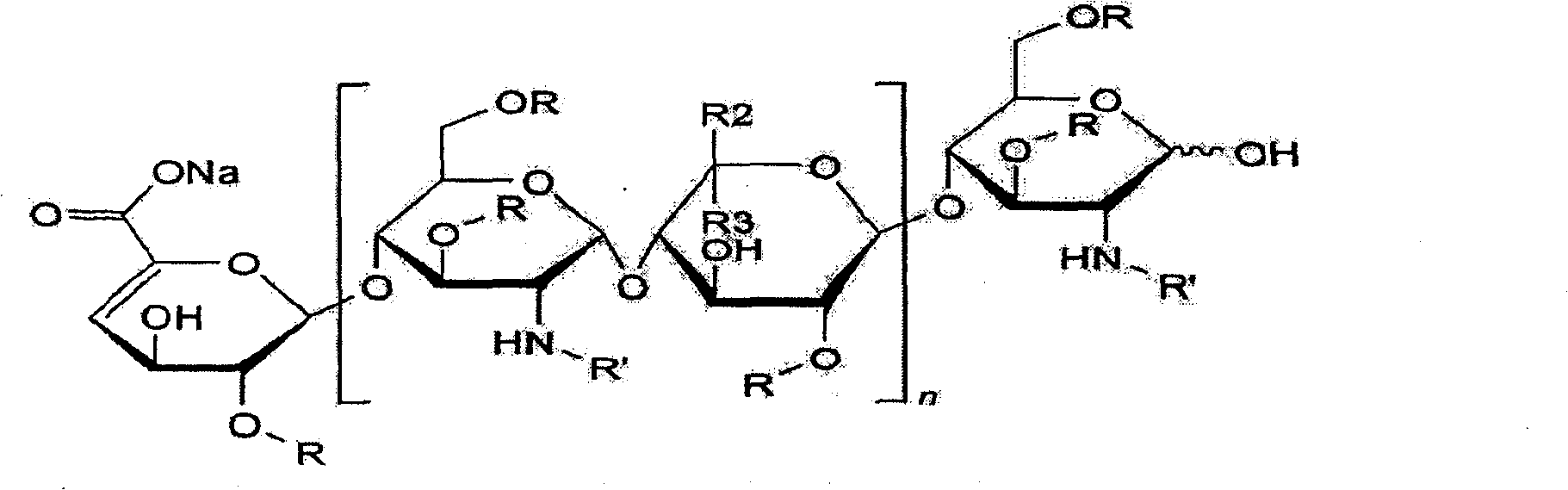
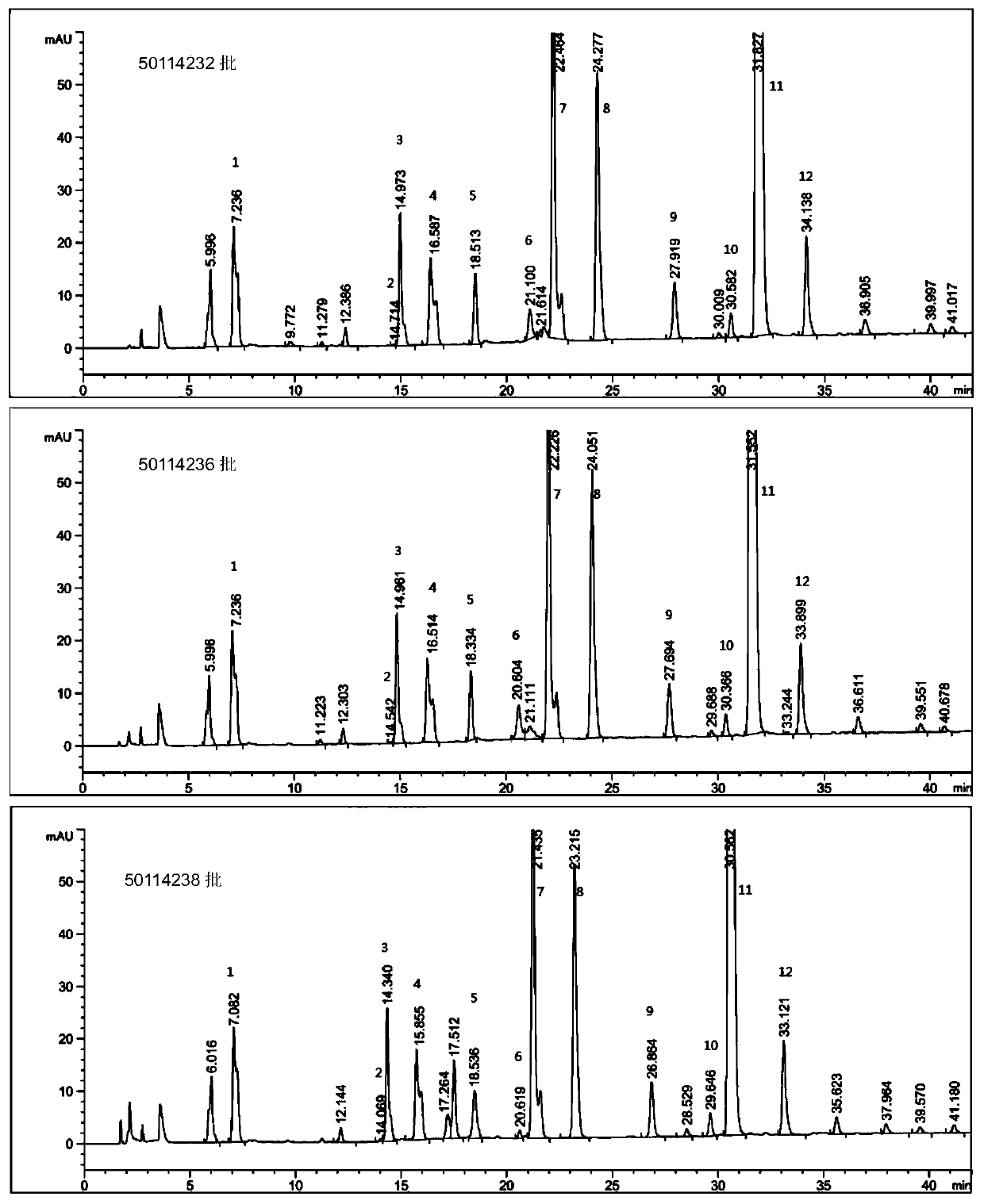
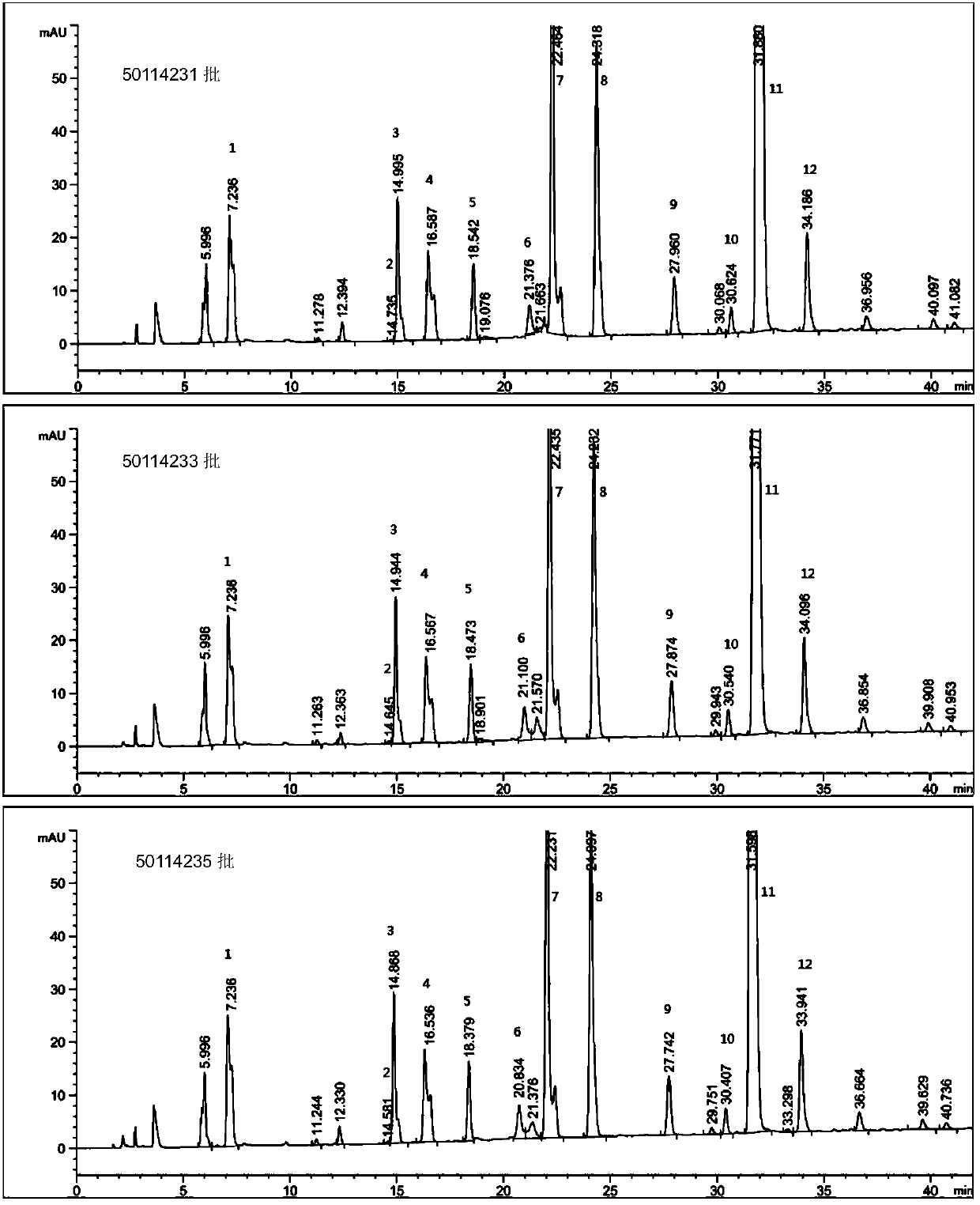
HILIC (hydrophilic interaction chromatography)-MRM (multiple-reaction monitoring) MS/MS (tandem mass spectrometry) detection method for basic constitutional units of low-molecular-weight heparin
ActiveCN106018597AHigh practical valueComponent separationBiological material analysisHydrophilic InteractionsHydrolysis
The invention relates to an HILIC (hydrophilic interaction chromatography)-MRM (multiple-reaction monitoring) MS / MS (tandem mass spectrometry) detection method for complete degradation products of low-molecular-weight heparin. Original reducing end and non-reducing end of enoxaparin sodium are identified by reducing the reducing end of enoxaparin sodium and adopting hydrolysis with hydrogen peroxide. Quantitative analysis is performed on all constitutional units with HILIC-MRM MS / MS, quantification is particularly performed on special structures with lower content, and characterization is performed on the low-molecular-weight heparin.
Owner:SHANDONG UNIV
Method for producing and purifying enoxaparin sodium
The invention relates to a method for producing and purifying enoxaparin sodium. The finished enoxaparin sodium which satisfies the pharmacopeia standards can be obtained through carrying out centrifugal separation and alcohol precipitation purification. The method is high in yield, simple and convenient to operate and good in product clarity, and is capable of satisfying the requirements of the pharmaceutical industry.
Owner:SUZHOU ERYE PHARMA CO LTD
Synthetic method of affinity precipitation medium and application thereof to preparation of enoxaparin sodium
The invention relates to a synthetic method of affinity precipitation medium and application thereof to preparation of enoxaparin sodium. The preparation method is as below: salinizing heparin with benzethonium chloride salt in a heparin sodium solution purified by the affinity precipitation medium; esterifying the heparin benzethonium chloride to generate heparin benzyl ester; carrying out depolymerization on heparin benzyl ester in alkaline conditions; the precipitating by ethanol to obtain an enoxaparin sodium crude product; and oxidizing for decolorization by hydrogen peroxide to obtain the enoxaparin sodium fine product. The invention uses crude heparin sodium as a raw material and employs the affinity precipitation medium for purification, so as to effectively remove impurities bonded or non-boned with the heparin, ensure integrity of the natural structure of heparin, increase the activity yield of heparin and provide the basis for the stability and controllability of the subsequent preparation process of the enoxaparin sodium. The invention reduces the production cost, improves production efficiency, and is more suitable for industrial production.
Owner:江西浩然生物制药有限公司
Enoxaparin sodium compound and preparation method thereof
The invention provides an enoxaparin sodium compound and a preparation method thereof. Liquaemin and benzethonium chloride are used as raw materials, sodium chloride is used as a neutral reaction medium and a microwave solid-phase synthesis method is carried out to prepare heparin quaternary ammonium salt.
Owner:SUZHOU ERYE PHARMA CO LTD
Method for preparing and purifying enoxaparin sodium
The invention discloses a method for preparing and purifying enoxaparin sodium. After a crude enoxaparin sodium product is subjected to centrifugal separation and alcohol precipitation impurity removal, the finished enoxaparin sodium product meeting pharmacopeia standards can be obtained. The method is high in yield and easy and convenient to implement, and the product is good in clarity and capable of meeting requirements of the pharmaceutical industry.
Owner:QINGDAO JIULONG BIO PHARMA
Heparanase composition capable of complete specific enzymolysis of enoxaparin sodium and application of heparanase composition
InactiveCN104792896AReduce the introductionSolve the problem of 1,6-anhydride disaccharide analysisComponent separationStrength propertiesActivity ratiosImpurity
The invention provides a heparanase composition capable of complete specific enzymolysis of enoxaparin sodium. The heparanase composition is a mixture of heparanase II and heparanase III, wherein the enzyme activity ratio of heparanase II to heparanase III is 3: 1. According to the invention, the heparanase composition can realize the complete specific enzymolysis of enoxaparin sodium and is reduced to obtain 1, 6-anhydride derivatives, and the contents are in an acceptable range; the heparanase composition is free from heparanase I to reduce the introduction of enzyme impurities, so that the method is more reliable, is an important supplement for several pharmacopoeia methods, has high practical use value and significance.
Owner:SUZHOU RONGXI BIOTECH CO LTD
RP-IP-HPLC (reverse-phase ion-pair high-performance liquid chromatography) method for collecting enoxaparin oligosaccharide
ActiveCN104483418AHigh yieldHigh purityComponent separationESI mass spectrometryMass Spectrometry-Mass Spectrometry
The invention belongs to the field of medicine, and relates to an RP-IP-HPLC (reverse-phase ion-pair high-performance liquid chromatography) method for collecting enoxaparin oligosaccharide. The method comprises the following steps: (a) a tetrose component mixture is collected from enoxaparin, wherein an enoxaparin sodium solution with the concentration of 50-70mg / ml is separated with high-resolution gel permeation chromatography, and the tetrose component mixture is obtained; (b) single tetrose components are collected from the tetrose component mixture with RP-IP-HPLC, wherein the tetrose component mixture is taken and made into a water solution with the concentration of 10mg / ml and is pre-separated with the reverse-phase ion-pair high-performance liquid chromatography, the pre-separated tetrose components are made into water solutions of 1mg / ml respectively and then are separated and purified with the reverse-phase ion-pair high-performance liquid chromatography; (c) the collected single tetrose components are made into the water solutions of 1mg / ml and are subjected to structural analysis through ESI-MS (electrospray ionization mass spectrometry).
Owner:SHENZHEN TECHDOW PHARM CO LTD
Method for preparing enoxaparin sodium through heparin benzyl ester
The invention relates to a method for preparing enoxaparin sodium through heparin benzyl ester. The method comprises the following steps of preparing sodium hydroxide with purified water to be a solution with the concentration being 0.06 to 0.2mol / L, and heating to be 62 DEG C, wherein the weight of the purified water is 20 to 30 times of the weight of the heparin benzyl ester; adding the sodium hydroxide solution with the temperature of 62 DEG C and the heparin benzyl ester for twice, and reacting for 1 to 4 hours at the temperature of 62 DEG C; cooling to room temperature, adding hydrochloric acid for adjusting pH(potential of Hydrogen) to be neutral, adding sodium chloride, filtering through a filter membrane, alcohol-precipitating and drying to obtain the enoxaparin sodium. According to the method provided by the invention, the concentration of the sodium hydroxide solution is adjusted according to the esterification rate of the benzyl ester, and the product 1,6-cyclo is ensured to keep between 15 percent to 25 percent specified by Pharmacopeia EP8.0 through controlling sequences of material adding and temperature rising, alkalifying and degrading for twice, and regulating degradation time during a degradation process, so that the stability of a pharmacological function is ensured.
Owner:苏州正济药业有限公司
Preparation method for improving clarity of enoxaparin sodium
The invention discloses a preparation method for improving the clarity of enoxaparin sodium. The method comprises the following steps: dissolving a crude product of enoxaparin sodium by adopting sodium chloride with concentration of more than 3.5%(w / v), ionizing quaternary ammonium salt bonded with the crude product of enoxaparin sodium through replacement reaction, and precipitating out enoxaparin sodium by using high-quantity ethanol or methanol. Compared with the prior art, the preparation method provided by the invention is easy and feasible, the product yield is high, and the clarity is relatively good.
Owner:山东万邦赛诺康生化制药股份有限公司
Refining method of enoxaparin sodium
The invention belongs to the technical field of medicine and relates to a refining method of enoxaparin sodium. The refining method solves the problems of the prior art, utilizes an enoxaparin sodium crude product as a raw material, realizes combination of hydrogen peroxide oxidation decoloring and methanol grading alcohol precipitation, and adopts a two-step decoloring and methanol grading alcohol precipitation method. The decolored agent obtained by the refining method is basically colorless, colored impurities can be effectively removed and an enoxaparin sodium finished product is obtained by spray drying. The refining method realizes synthesis of the enoxaparin sodium finished product satisfying the requirement EP 7.0. The refining method has product collection easiness and is suitable for industrial expanded production. Through integral cooperation of decoloring, grading alcohol precipitation and spray drying, product clarity is less than or equal to that of a turbidimetric liquid 0.5, European pharmacopoeia EP 7.0 requirements are satisfied, production processes are simple, product collection is easy and the refining method is provided for industrial production.
Owner:JIANGSU WANBANG BIOPHARMLS +1
A process for preparing enoxaparin sodium
ActiveCN104086674BHigh activityImprove product qualityEthanol precipitationAnion-exchange chromatography
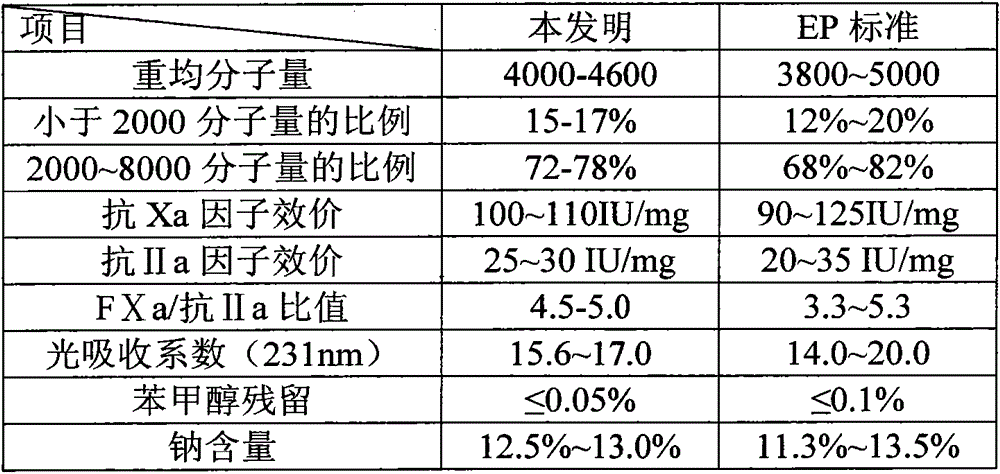
The invention relates to a process for preparing enoxaparin sodium, comprising: S1: preparation of heparin-benzethonium chloride salt S2: preparation of heparin benzyl ester; S3: cracking of heparin benzyl ester S4: preparation of enoxaparin sodium Decolorization; S5: anion exchange chromatography: collect the eluate, detect the molecular weight distribution as the weight average molecular weight of 4000-4600, and the proportion of molecular weight of 2000-8000 is 72‑78%; S6: freeze-drying: alcohol precipitation, sterilization, freeze-drying , to obtain the enoxaparin sodium. The method obtains high-yield and high-purity enoxaparin sodium products by using anion-exchange resin chromatography, etc., and the process has a high degree of automation and convenient operation, which provides a strong guarantee for increasing production capacity and is suitable for large-scale production.
Owner:CHANGZHOU QIANHONG BIOPHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com