A process for preparing enoxaparin sodium
A technology of enoxaparin sodium and heparin sodium, which is applied in the fields of medicine and chemistry, can solve the problems of low degree of automation, time-consuming, laborious, etc., and achieve the effect of convenient operation, high degree of automation, and increased production capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Process for preparing enoxaparin sodium
[0026] ①Preparation of heparin-benzethonium chloride salt: Dissolve 4kg of heparin sodium in 40kg of purified water, dissolve 4-6kg of benzethonium chloride in 50L of purified water, and slowly add heparin sodium solution to benzethonium chloride with stirring at room temperature In the ammonium solution, continue to stir at room temperature for 1 to 5 hours after the addition is complete, centrifuge the precipitate, wash it repeatedly with purified water, and centrifuge until the filtrate meets 10% silver nitrate solution without turbidity, and vacuum dry the precipitate at 50°C to 60°C After 24 hours, heparin benzethonium chloride salt with a water content of less than 5% was obtained.
[0027] ②Preparation of heparin benzyl ester: dissolve the heparin sodium benzethonium chloride salt obtained in the previous step in a weight ratio of 1:1 (heparin sodium benzethonium chloride salt: dichloromethane) in dichlorometha...
Embodiment 2
[0032] Example 2: Process for preparing enoxaparin sodium
[0033]①Preparation of heparin-benzethonium chloride salt: Dissolve 4kg of heparin sodium in 40kg of purified water, dissolve 5-8kg of benzethonium chloride in 50L of purified water, slowly add heparin sodium solution into benzethonium chloride under stirring at room temperature In the ammonium solution, continue to stir at room temperature for 1 to 5 hours after the addition is complete, centrifuge the precipitate, wash it repeatedly with purified water, and centrifuge until the filtrate meets 10% silver nitrate solution without turbidity, and vacuum dry the precipitate at 50°C to 60°C After 24 hours, heparin benzethonium chloride salt with a water content of less than 5% was obtained.
[0034] ② Preparation of heparin benzyl ester: dissolve the heparin sodium benzethonium chloride salt obtained in the previous step in dichloromethane in a weight ratio of 1: 2 to 3 (heparin sodium benzethonium chloride salt: dichlorom...
Embodiment 3
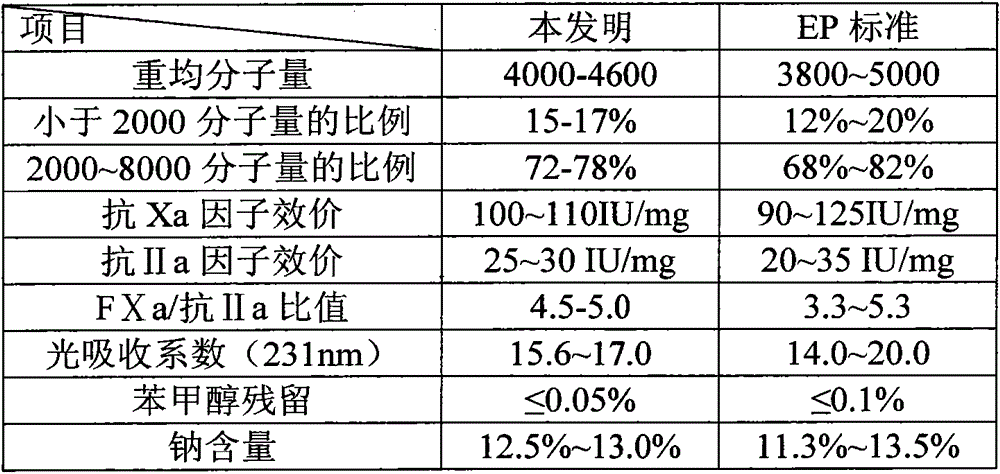
[0040] The invention creates a process for preparing enoxaparin sodium through anion exchange chromatography technology. Heparin sodium is sequentially subjected to benzethonium chloride salification, benzyl esterification, alkaline degradation, oxidative decolorization, filtration to remove impurities, and the filtrate is subjected to anion exchange chromatography to obtain enoxaparin sodium products whose average molecular weight distribution meets the requirements. The present invention adopts anion exchange resin, distributes and collects eluent, and prepares a stable and controllable quality, high-purity, high-activity enoxaparin product, and the quality is obviously higher than the standard of the current EP Pharmacopoeia (see the table below).
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com