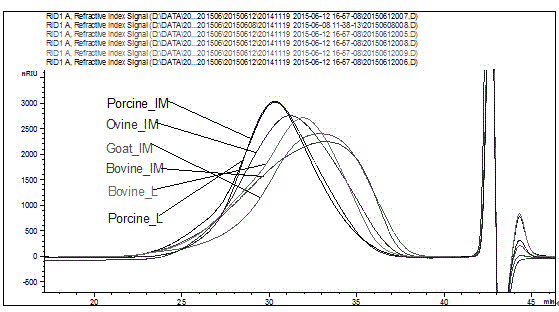
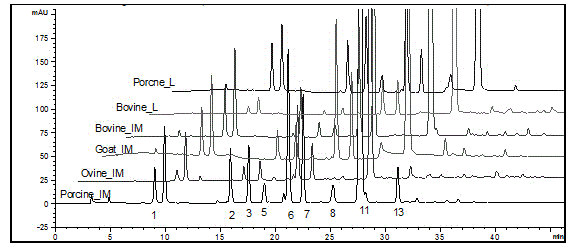
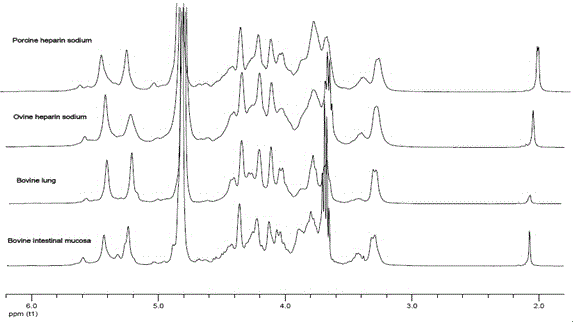
Sheep enoxaparin sodium compound preparation method, compound and application of compound
An enoxaparin sodium and compound technology, applied in the field of medicine and biology, can solve the problems of high process and technical requirements, product similarities and differences, no comparison of physical chemical and biological properties, etc., achieves huge economic potential, promotes effective utilization, raw materials easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Sheep intestinal mucosal heparin sodium was purified and prepared in Suzhou Rongyan Biotechnology Co., Ltd. according to the general heparin purification method well known in the art. After testing, the anticoagulant activity of the sheep plasma method is 166.8 units per mg after being dried, and the solution of 50,000 units of the product dissolved in 10 ml of water is clear and the chroma is not deeper than the No. 5 standard color.
[0117] Accurately weigh 50 grams of the sheep intestinal mucosa heparin sodium and dissolve it in 500 milliliters of water; in addition, dissolve 125.0 grams of benzethonium chloride in 500 milliliters of water to prepare a clear aqueous solution of benzethonium chloride; Thoxonium chloride aqueous solution was added dropwise to the heparin aqueous solution, and the dropwise addition was completed within 30 minutes, and the stirring was continued for 2 hours. Centrifuge at 6,000 rpm for 5 minutes with a high-speed centrifuge, resuspend t...
Embodiment 2
[0122] Take 2.0 kg of sheep intestinal mucosal heparin sodium prepared by Suzhou Biotechnology Co., Ltd., its sheep plasma method anticoagulant activity is 171.5 units per mg after drying, and the solution of 50,000 units of the product dissolved in 10 ml of water is clear and the color is not Deeper than No. 5 standard color. The process of preparing sheep enoxaparin sodium is the same as that in Example 1, except for the difference in the amount of reagent used. Finally, 1.16Kg of sheep enoxaparin sodium was obtained, the weight yield was 58.0% based on the initial feeding, and the loss on drying was 3.4%. ; 1.0 grams of product dissolved in 10 ml of water solution, clear and not darker than No. 6 standard color; 1.0 g of product dissolved in 10 ml of water solution, pH is 7.4; sodium content is 11.5% after drying; nitrogen content After drying, it is 2.2%; the aqueous solution has a maximum absorption at a wavelength of 232 nanometers, and the characteristic absorption at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com