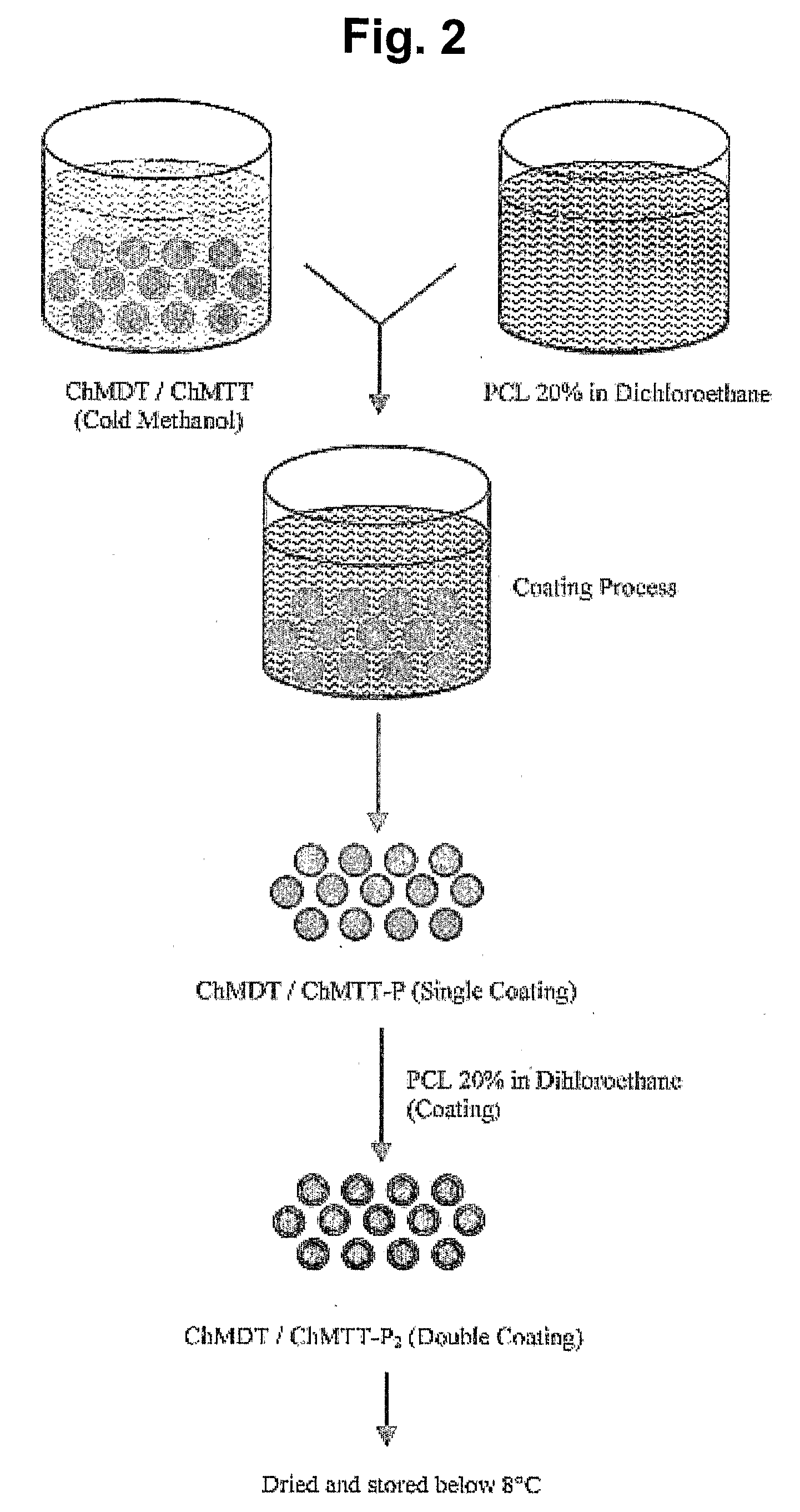
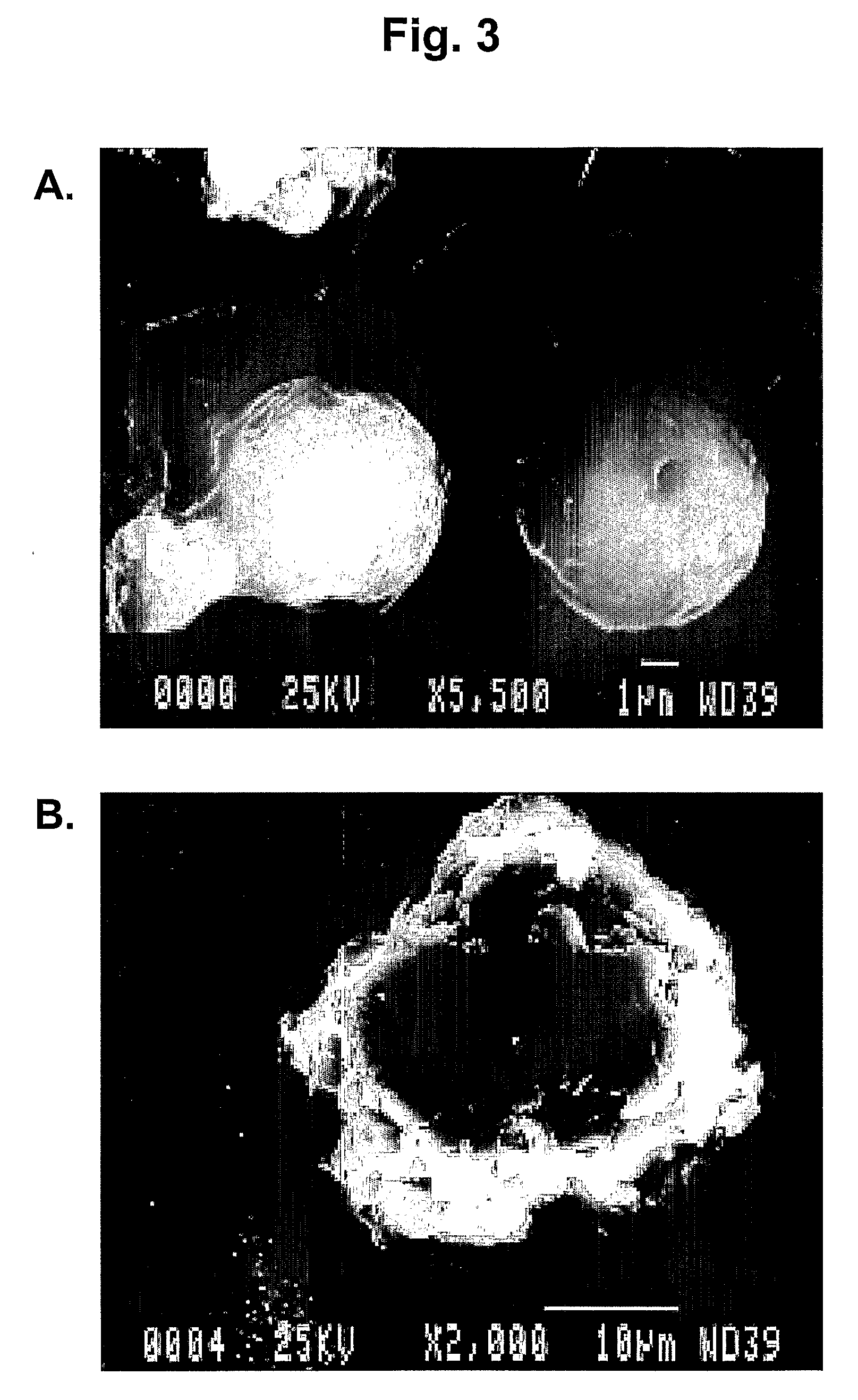
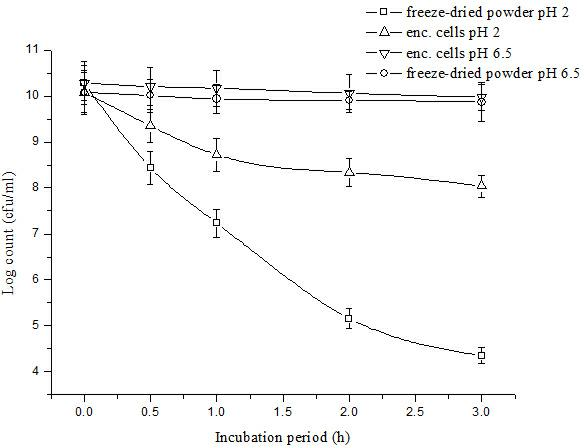
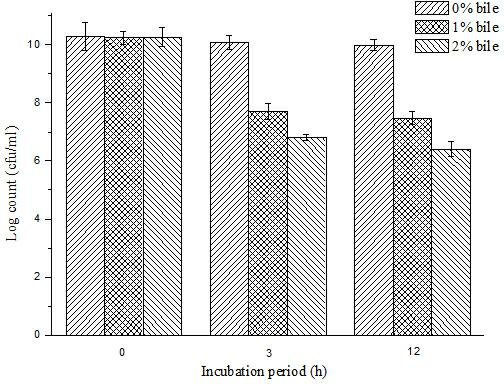
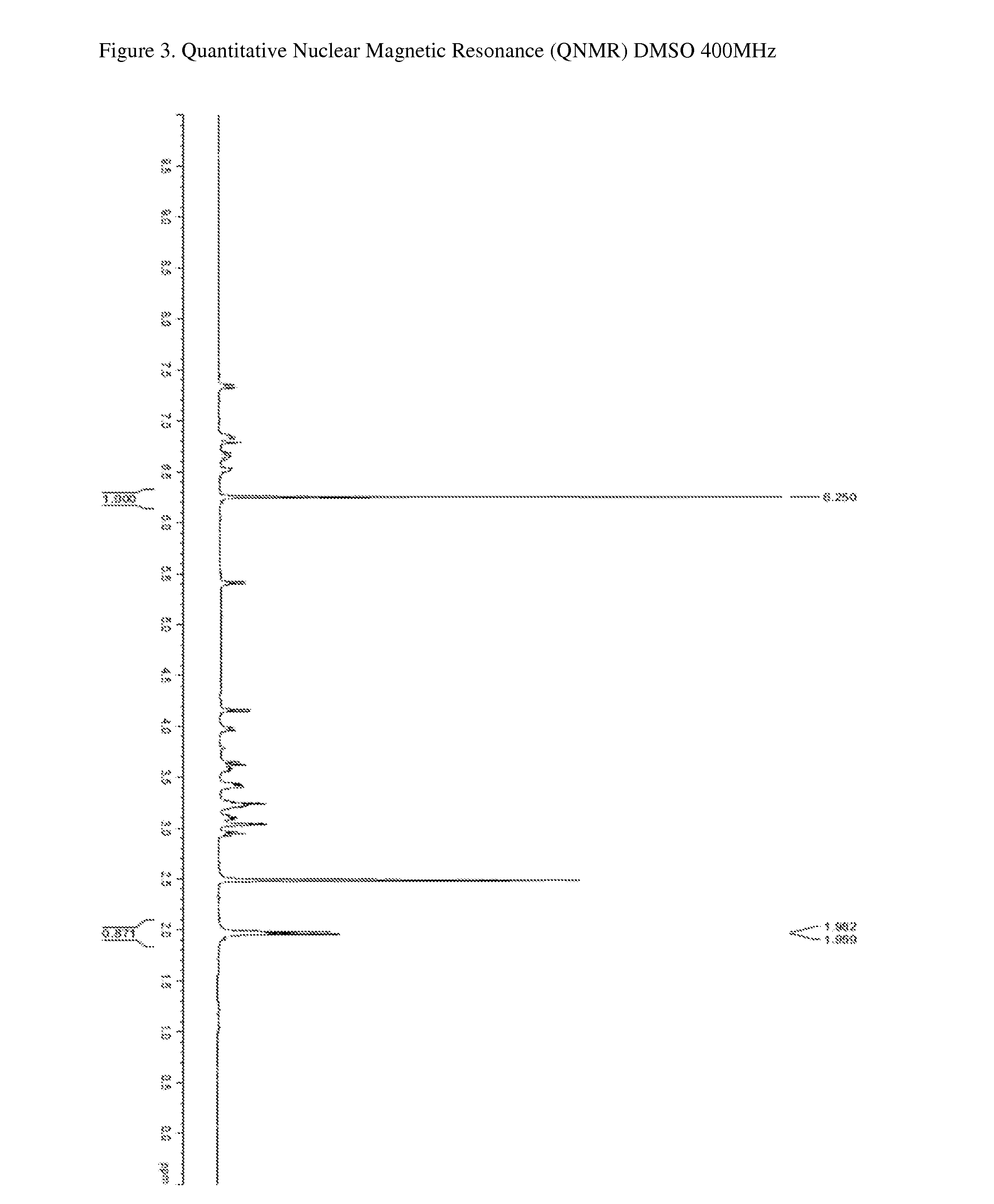Patents
Literature
145 results about "Intestines mucosa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions Containing Enriched Natural Crocin and/or Crocetin, and Their Therapeutic or Nutraceutical Uses
ActiveUS20140141082A1Reduce eliminate undesirable componentEasy to useBiocideHydrocarbon active ingredientsDiseasePhytochemical
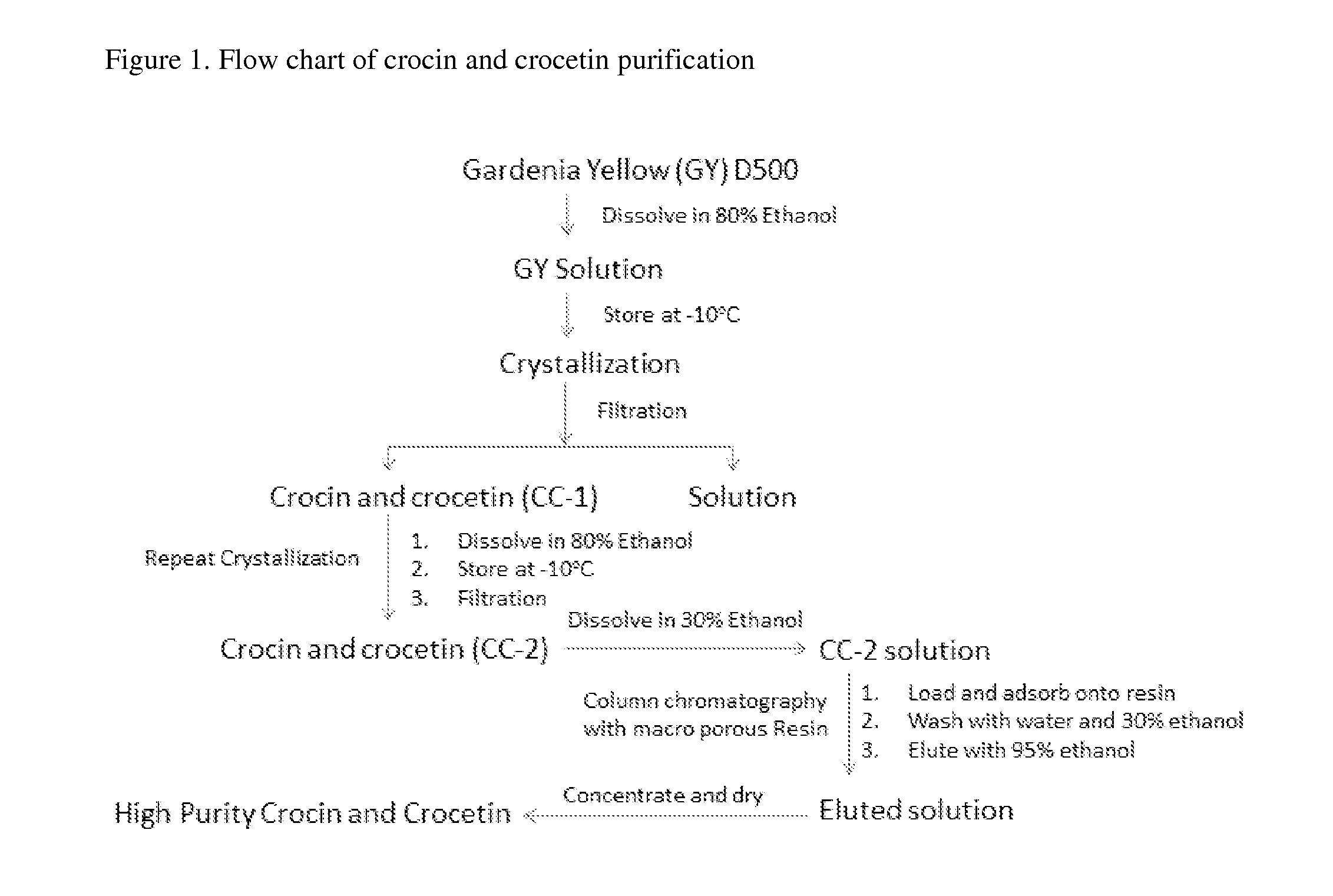
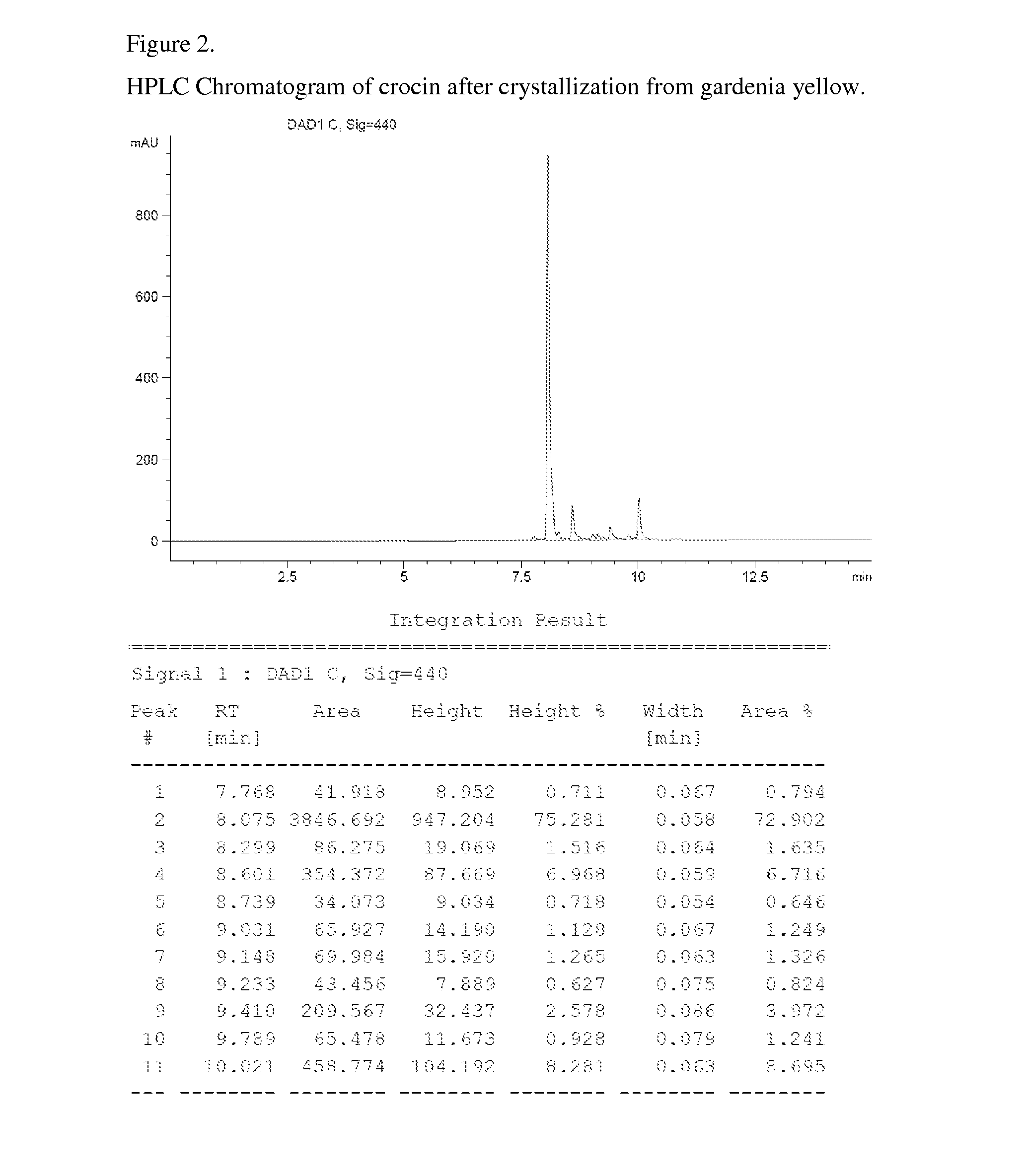
The invention relates to unique compositions containing enriched and purified natural crocin and / or crocetin for prevention and / or treatment of cancers and other conditions and diseases. Compositions comprise mainly enriched or purified natural crocin or crocetin or combination of both and possible other active phytochemicals. A composition is used as functional food, drink, dietary supplement, or therapeutic dosage to a human orally or through other appropriate way (parenteral, percutaneous, rectal, mucosal, intranasal or topical administration). A method of natural crocin and crocetin enriching and purification is revealed.
Owner:GAO SONG
Microparticles and Nanoparticles for the Transmucosal Delivery of Therapeutic and Diagnostic Agents
InactiveUS20080102114A1Easy to transportEasy to demonstrateOrganic active ingredientsPeptide/protein ingredientsMicrosphereMicroparticle
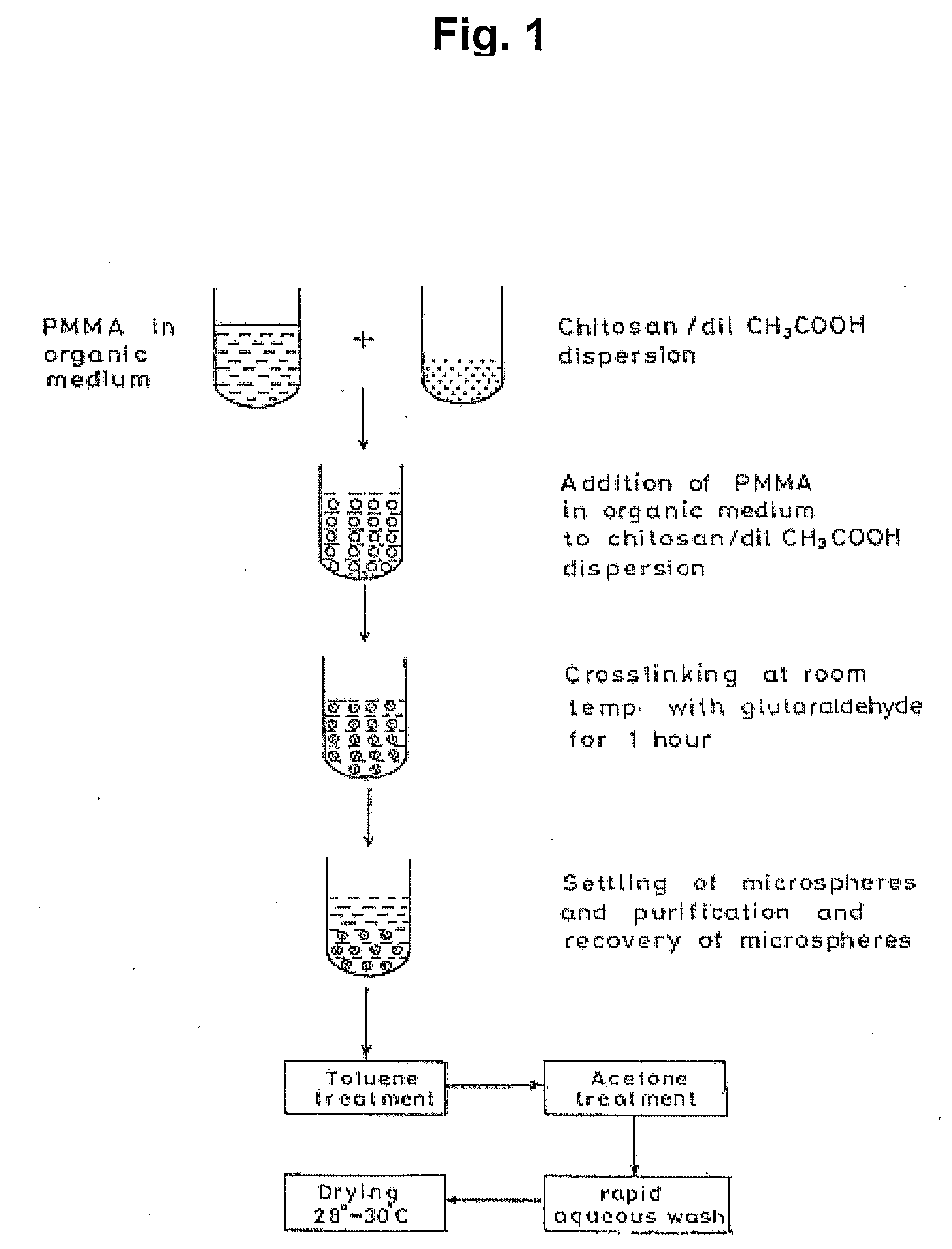
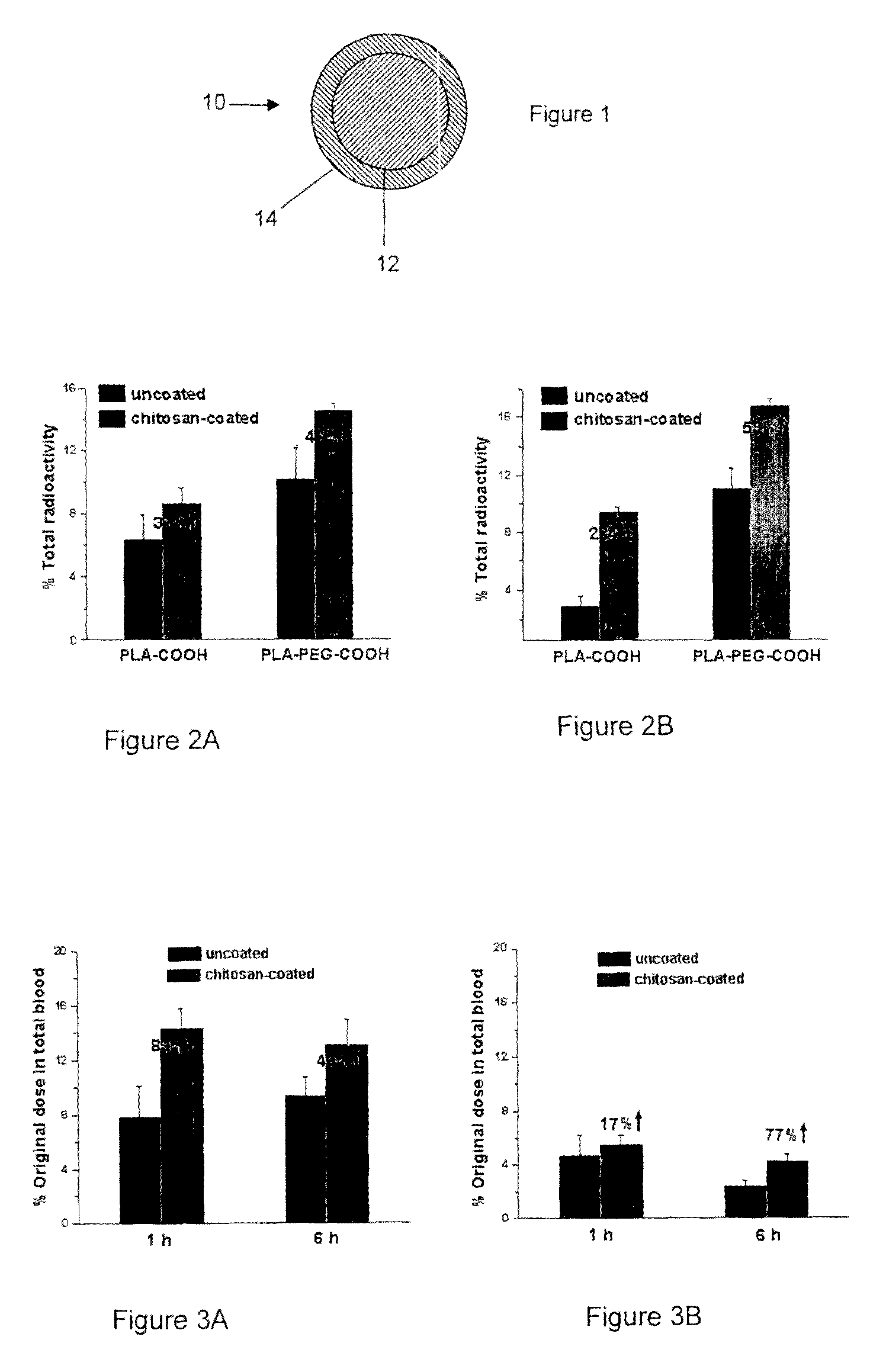
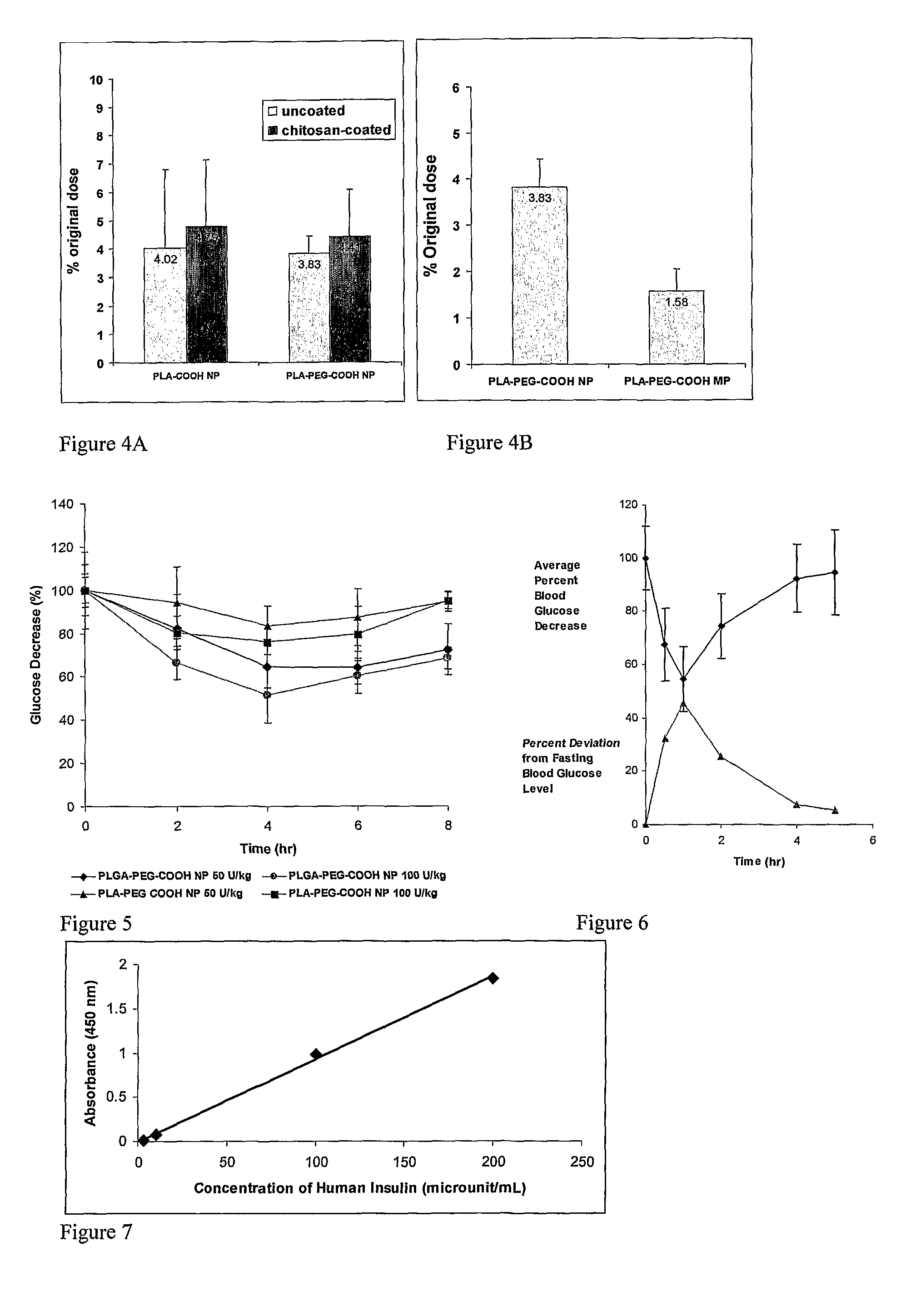
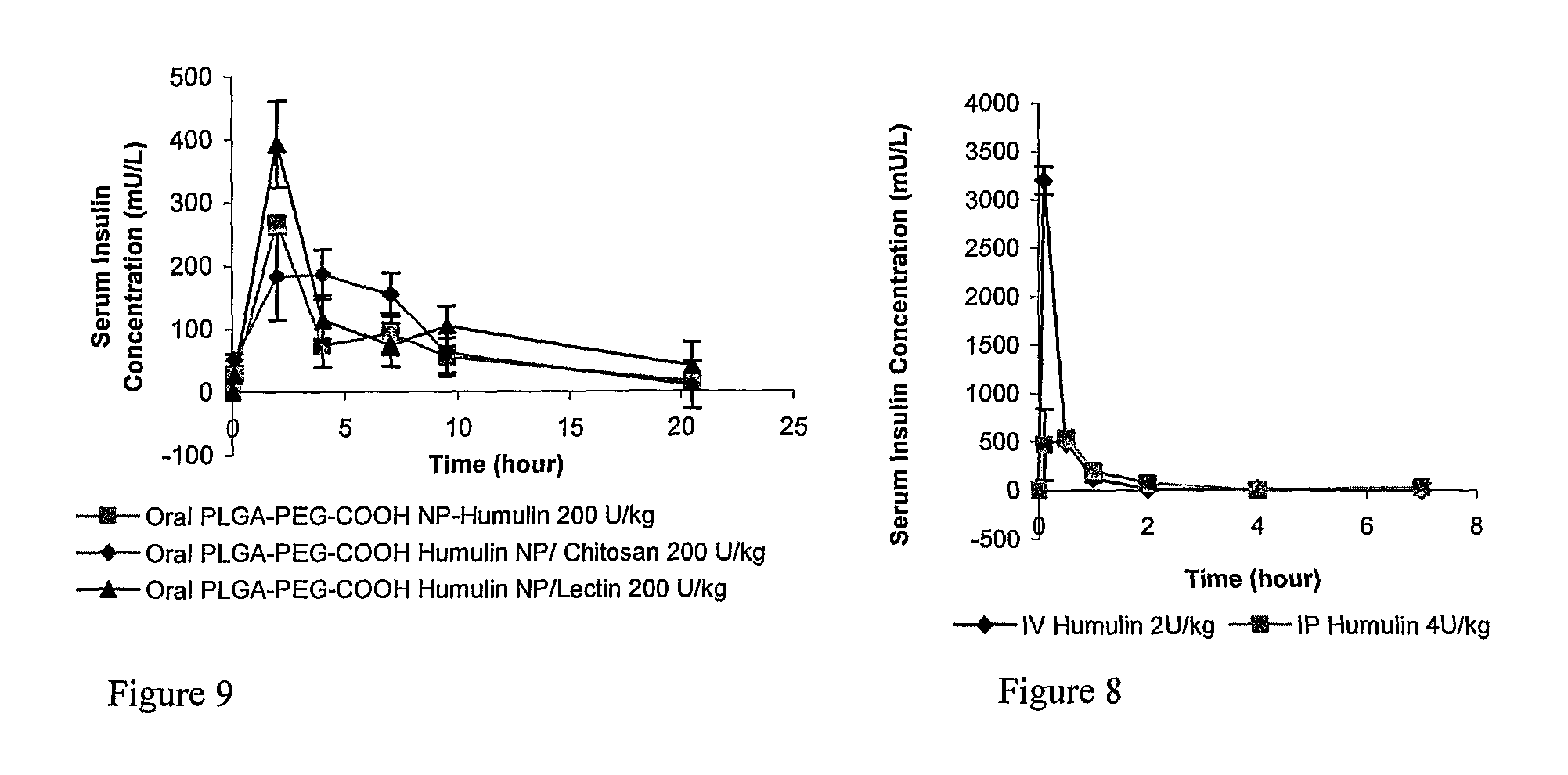
The invention relates to compositions and methods for the administration of therapeutic and / or diagnostic agents such as polypeptides to a mammal, and in particular, compositions suitable for oral administration. The invention provides polymeric particles, and in particular, nano / microparticles such as, but not limited to, microspheres and nanospheres, as well as methods of synthesizing them. The invention also provides methods of increasing the serum concentration of a therapeutic agent such as a polypeptide by orally administering polymeric particles comprising the therapeutic agent. The compositions of the invention allow the absorption of polypeptides through intestinal mucosa and intestinal cells and into the bloodstream of a mammal. The invention further provides a method of treating type II diabetes through the oral administration of compositions comprising insulin and also provides a related glucose-responsive insulin delivery system.
Owner:KORITALA PANDURANGA RAO +1
Bifidobacterium microcapsule and preparing method thereof
ActiveCN102210659AResistant to gastric acidBile salt resistantMetabolism disorderBacteria material medical ingredientsFreeze-dryingHigh survival rate
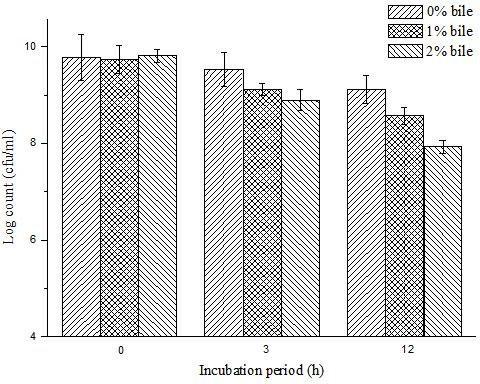
The invention specifically relates to a bifidobacterium microcapsule and a preparing method thereof. The bifidobacterium is obligatorily anaerobic and very sensitive to oxygen, PH, temperature, humidity, and other adverse external environment, thereby being very hard to remain activity during production, storage and transport; besides, if taken orally in a form of dry bifidobacterium powder, the bifidobacterium cannot tolerate low-pH value gastric acid, bile salt, and other environments, so a purpose that massive survived bifidobacteria arrive at an intestinal tract and colonise on the intestinal mucosa is hard to be guaranteed. The preparing method disclosed by the invention comprises the following steps of: adding freeze-dried bifidobacterium powder into a sodium alginate solution and then mixing in soybean oil, emulsifying the mixed solution and standing; centrifugally collecting micro-capsules; and drying the micro-capsules by using a vacuum freeze-drying technology. The bifidobacterium microcapsule disclosed by the invention has the advantages of gastric acid resistance, bile salt resistance and entericsolubility, high survival rate of bifidobacterium, simple preparation method, strong practicality, convenience for industrial production, and excellent storage stability and solves the problem of short storage period of probiotics preparations.
Owner:SHAANXI GIANT BIOTECHNOLOGY CO LTD
Intestinal mucosa protection and restoration type enteral nutritive product and preparation method thereof
ActiveCN103549410APromote peristalsisPrevent constipationVitamin food ingredientsFood ingredient functionsBiotechnologyNutrition
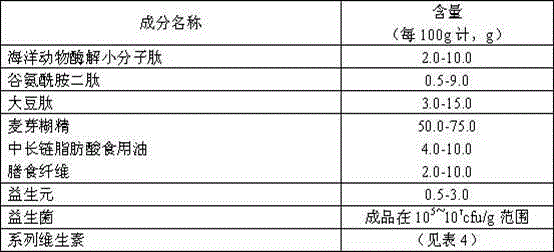
The invention belongs to a nutritive food production field, concretely an intestinal mucosa protection and restoration type enteral nutritive product and a preparation technology thereof, and provides a product capable of protecting intestinal mucosa, promoting restoration of the intestinal mucosa after injury, preventing and mitigating patient constipation, and providing equalization nutrition support. The nutritive product is prepared from micromolecule peptides of sea animals, glutamine dipeptide, soybean peptide, maltodextrin, dietary fiber, and medium- and long-chain triacylglycerol oil, etc., is blended with composite vitamins, prebiotics and probiotics according to nitrogen and heat requirements recommended to medium stress patients, can protect the intestinal mucosa and promote restoration of the intestinal mucosa, and promotes intestinal colonization of beneficial bacteria, thereby inhibiting harmful bacteria proliferation, reducing endotoxin generation, preventing movement of endotoxin, and providing a nitrogen source, carbohydrate, fat and vitamins required by a human body in a pathologic period. The product is suitable for a patient with intestinal mucosa injury caused by a plurality of factors like wound, great surgery operation and tumour chemotherapy, is safe and has no toxic side effect, and can be prepared to be electuary or functional nutritive injectable suspension.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method for preparing intestine nutritive peptide by using wheat
ActiveCN103667408AAvoid Water AgglomerationAvoid efficiencyPeptide/protein ingredientsPeptide preparation methodsNutritionAlkaline proteinase
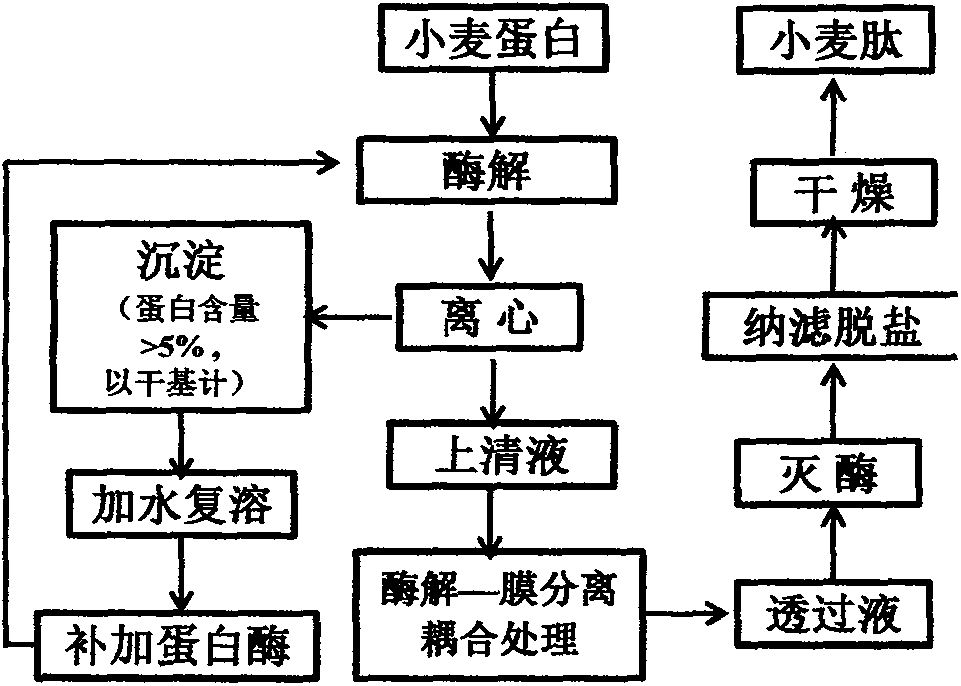
The invention provides a method for preparing an intestine nutritive peptide by using wheat, and belongs to the technical field of deep processing of wheat protein. The method comprises the following steps: (1) pre-mixing the wheat with polysaccharide and adding water for dispersion, so that wheat protein suspension liquid solubilized through the polysaccharide is obtained; (2) selecting protamex<TM> to perform restriction enzymolysis on the wheat protein step by step, wherein the substrate obtained in the later step is a precipitate produced through enzymolysis in the step (1), the times for enzymolysis are controlled to be 3-4, and when the protein content in the final precipitate is less than 5% (dry basis), the enzymolysis is stopped; (3) using a continuous enzymolysis-membrane separation coupling system for treating a supernatant centrifuged through enzymolysis in the above steps, adding a certain amount of alcalase in the system for enzymolysis for a certain time, and then desalting permeating liquid through nanofiltration, so that wheat oligopeptide with high glutamine is obtained. The wheat active peptide prepared through the method can alleviate injury degree of enteritis rat intestinal mucosa, and promote the synthesis of intestinal mucosa protein and DNA, and has an obvious efficacy of enteral nutrition.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Process for extracting heparin sodium efficiently from small intestines of pigs
The invention relates to the technical field of the production of heparin sodium, and provides a process for extracting heparin sodium efficiently from small intestines of pigs. According to the process, casings and intestinal mucosae are combined to prepare the heparin sodium, so that the utilization rate of the small intestines of the pigs is improved, the additional value of the small intestines of the pigs is increased, 0.1 billion international units of heparin sodium crude products are extracted only from about 1,400 small intestines of the pigs, the purity of the product is high, and the valence of the heparin sodium is more than 100 U / mg. Simultaneously, the residual casings can also be utilized effectively. Trypsin enzyme liquid is prepared from fresh porcine pancreas serving as raw materials and is used for extracting the heparin sodium, so that artificially-synthetic substances are reduced greatly, and the naturalness of the product is improved; and the heparin sodium is extracted from the trypsin enzyme liquid, so that peculiar smell generated in the production process can be reduced to the maximum degree, and environmental protection is facilitated.
Owner:HANGZHOU LONGYANG BIOTECH
Coated controlled release polymer particles as efficient oral delivery vehicles for biopharmaceuticals
A composition for delivering an active agent to a patient. The composition includes a polymer core encapsulating the active agent and a mucoadhesive coating disposed about the core. The polymer may include covalently linked poly(ethylene glycol) chains, and the mucoadhesive coating may be selected to facilitate transfer of the particle through the intestinal mucosa. A molecular weight and cross-link density of the polymer may be selected such that the polymer core will decompose in a predetermined time interval. The fraction of the dose of the drug entering the system at circulation during the predetermined time interval may be between about 0.25% and about 25%. The composition may be formulated as a plurality of nanoparticles or microparticles that are combined with a pharmaceutically acceptable carrier to produce an edible or inhalable drug product.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Feed additive capable of preventing animals from being harmed by mycotoxin in feed, production method of feed additive and animal feed
InactiveCN105639108AInhibition of reproductionAvoid breedingFood processingFood preservationSodium bicarbonateMycotoxin
The invention discloses a feed additive capable of preventing animals from being harmed by mycotoxin in a feed, a production method of the feed additive and an animal feed. The feed additive comprises the following components in parts by weight: 20-30 parts of sodium diacetate, 10-50 parts of glucose oxidase, 30-50 parts of montmorillonite and 25-65 parts of a carrier, wherein the carrier comprises the following components in parts by weight: 3-10 parts of sodium bicarbonate, 3-10 parts of corn starch and 3-10 pats of glucose. The feed additive is safe and has no toxic and side effects and has multiple functions of adsorbing and clearing mold, preventing the reproduction of mold, protecting intestinal mucosa and livers of animals and therefore preventing the harm of mycotoxin.
Owner:北京兴潮生物技术有限公司
Process for extracting heparin sodium from intestinal mucosa by alkaline protease method
The invention relates to the technical field of production of heparin sodium and provides a process for extracting heparin sodium from an intestinal mucosa by an alkaline protease method. The process mainly comprises the steps of performing enzymolysis, adsorbing, washing, eluting, precipitating and drying. According to the process, the production cycle is short, the products have high yield and purity, discharge of intestinal residue and waste water is reduced, and environmental protection is promoted. 100 million international units of heparin sodium crude products can be produced only by about 1,600 small intestines of pigs, and the valence of the heparin sodium is more than 110 U / mg.
Owner:HANGZHOU LONGYANG BIOTECH
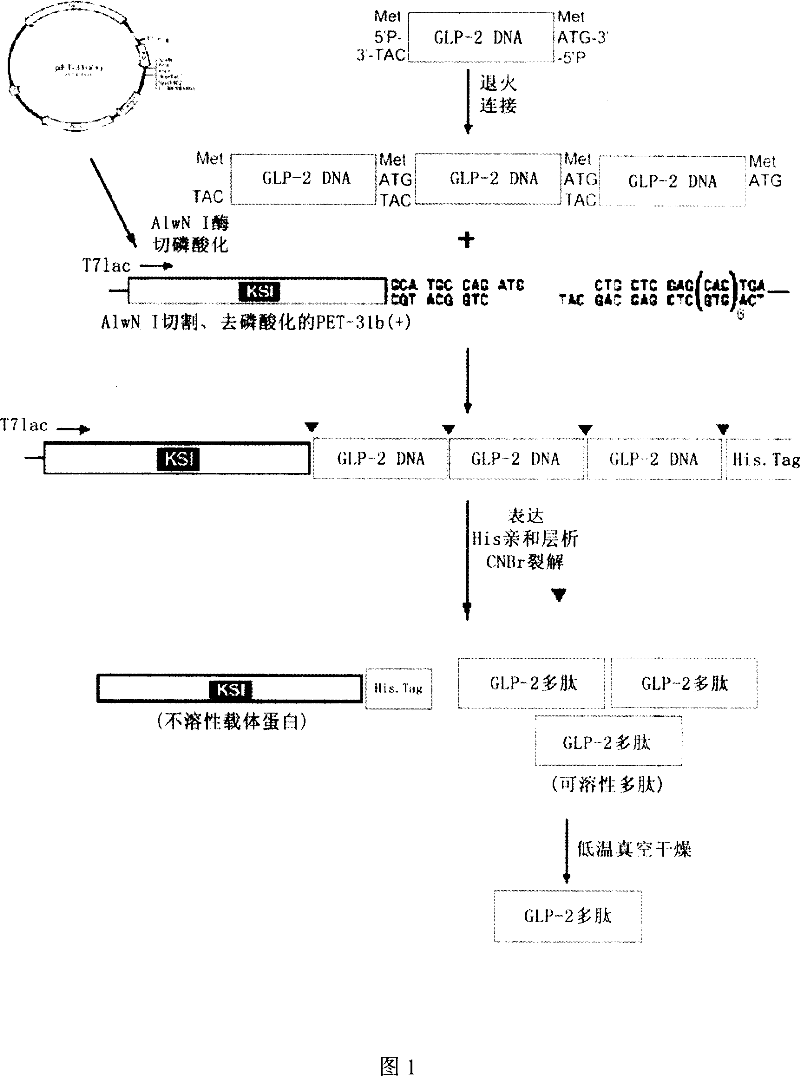
Method for recombinant production of pancreatic glucagons sample peptide-2
InactiveCN101041818AIncrease contentBacteriaPeptide/protein ingredientsPancreatic glucagonDrug biological activity
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Compositions containing enriched natural crocin and/or crocetin, and their therapeutic or nutraceutical uses
ActiveUS9211298B2Easy to useReduce and eliminate componentHydrocarbon active ingredientsHydroxy compound active ingredientsDiseasePhytochemical
The invention relates to unique compositions containing enriched and purified natural crocin and / or crocetin for prevention and / or treatment of cancers and other conditions and diseases. Compositions comprise mainly enriched or purified natural crocin or crocetin or combination of both and possible other active phytochemicals. A composition is used as functional food, drink, dietary supplement, or therapeutic dosage to a human orally or through other appropriate way (parenteral, percutaneous, rectal, mucosal, intranasal or topical administration). A method of natural crocin and crocetin enriching and purification is revealed.
Owner:GAO SONG
Application of butyric acid producing beneficial bacterium in preparing preparation for preventing and treating severe disease gut barrier injury and post-injury complication
InactiveCN101849969AMetabolism disorderBacteria material medical ingredientsParentucelliaPost injury
The invention relates to a new use of a butyric acid producing beneficial bacterium, in particular to one or more applications of the butyric acid producing beneficial bacterium in preparing a preparation for preventing and treating a severe disease gut barrier injury and post-injury complication, wherein the prevention and treatment of the gut barrier injury and post-injury complication of the severe disease is a prevention an treatment function on the gut barrier which is realized by producing the butyric acid in the intestinal tract, introducing the butyric acid into the intestinal tract, providing intestinal mucosa energy, promoting cell proliferation of the intestinal mucosa, restricting the cell apoptosis of the intestinal mucosa, improving the permeability of the intestinal mucosa and preventing and curing the intestinal mucosa carrier.
Owner:QINGDAO EASTSEA PHARMA +1
Application of lactobacillus reuteri in preventing and relieving ulcerative colitis
ActiveCN112646744AImprove toleranceReduce abundanceMilk preparationBacteriaUlcerative colitisDisease activity
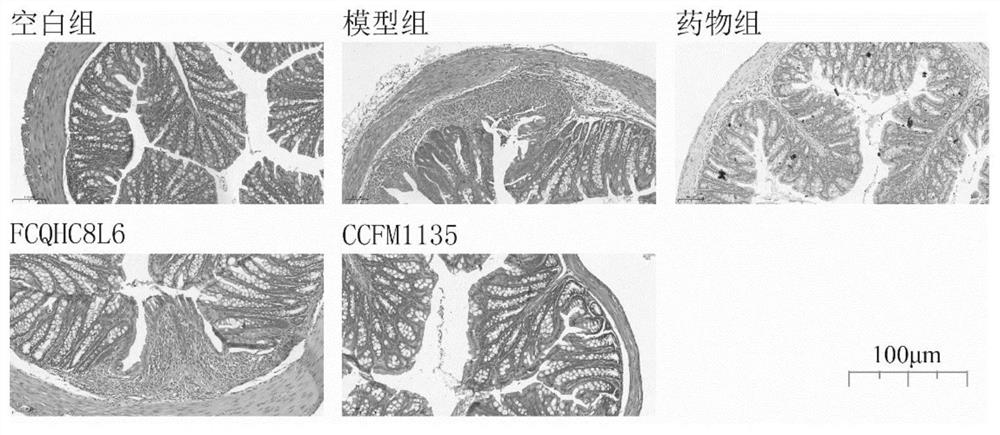
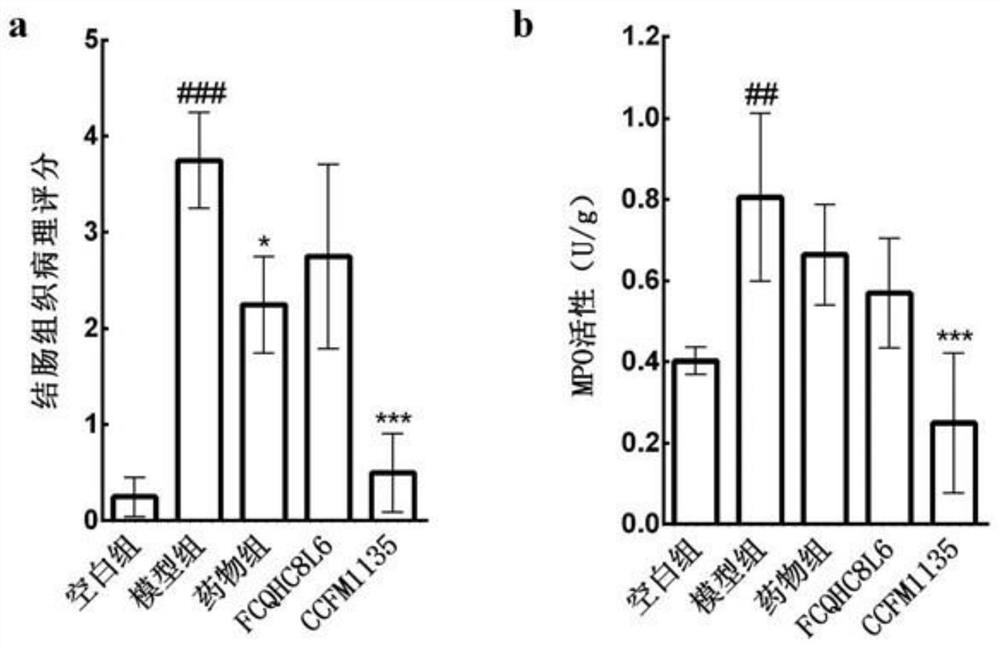
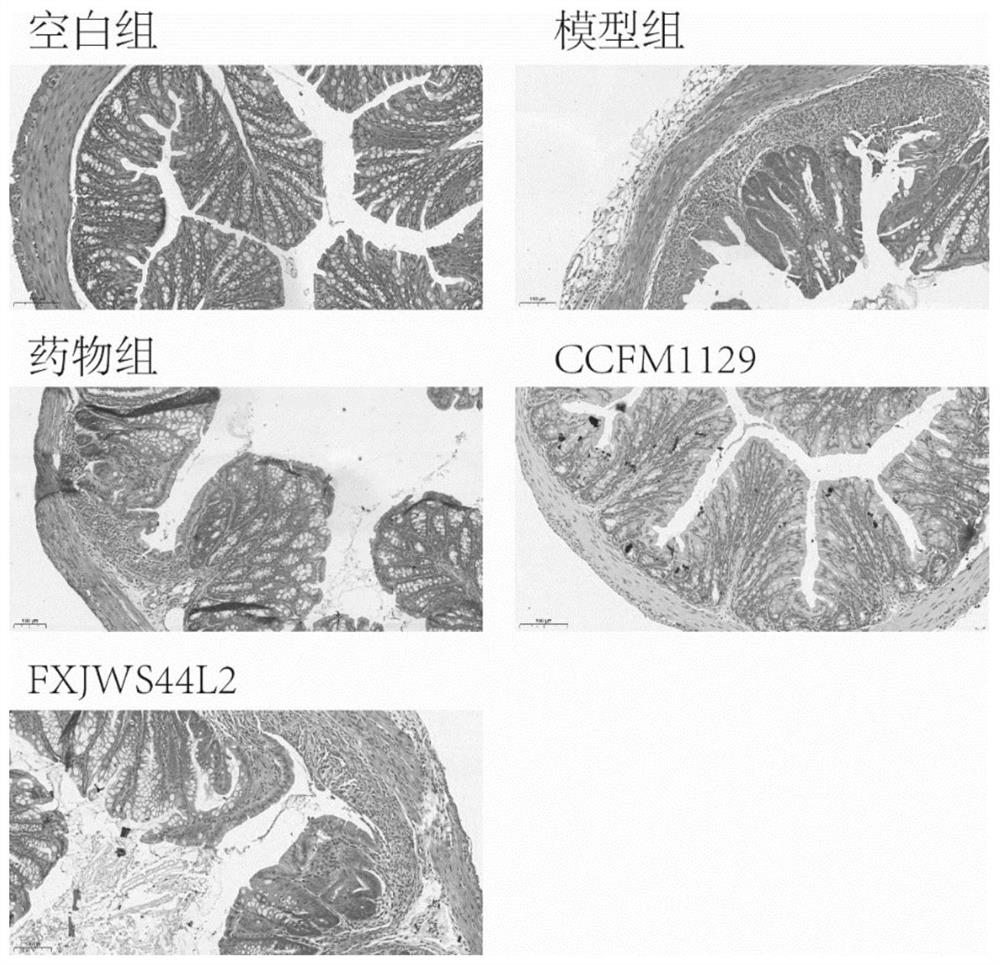
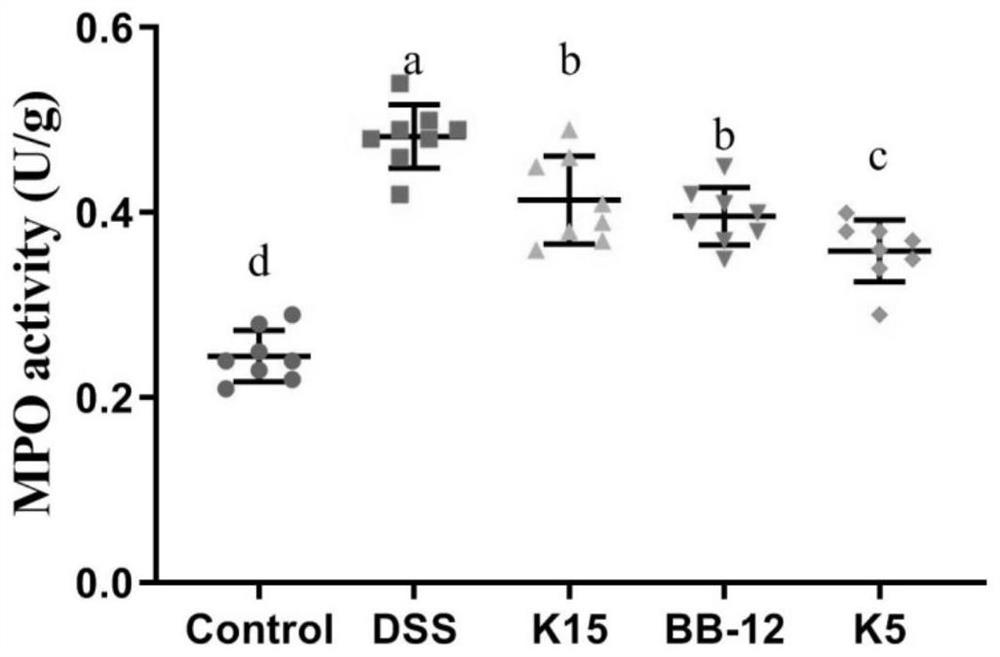
The invention discloses application of lactobacillus reuteri in preventing and relieving ulcerative colitis, and belongs to the technical field of functional microorganisms. Lactobacillus reuteri CCFM1135 can tolerate the gastrointestinal environment of a human body, significantly reduce the disease activity index during the ulcerative colitis, improve colon mucosal injury, reduce MPO activity, reduce the content of proinflammatory factors TNF-alpha, IL-6 and IFN-gamma in colon, up-regulate the transcription level of colon tight junction related proteins Claudin-3, ZO-1, ZO-2 and Occludin, up-regulate the transcription levels of colon antibacterial peptides Reg3g and Reg3b, improve the diversity of intestinal flora, and reduced the relative abundance of acinetobacter in excrement.
Owner:JIANGNAN UNIV
Lactobacillus plantarum for relieving ulcerative colitis and application thereof
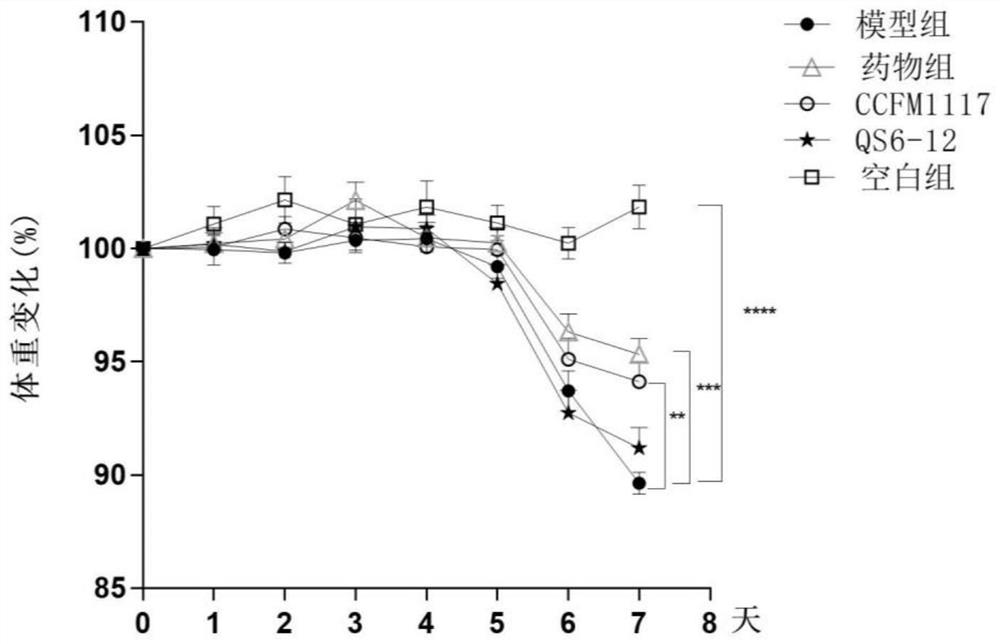
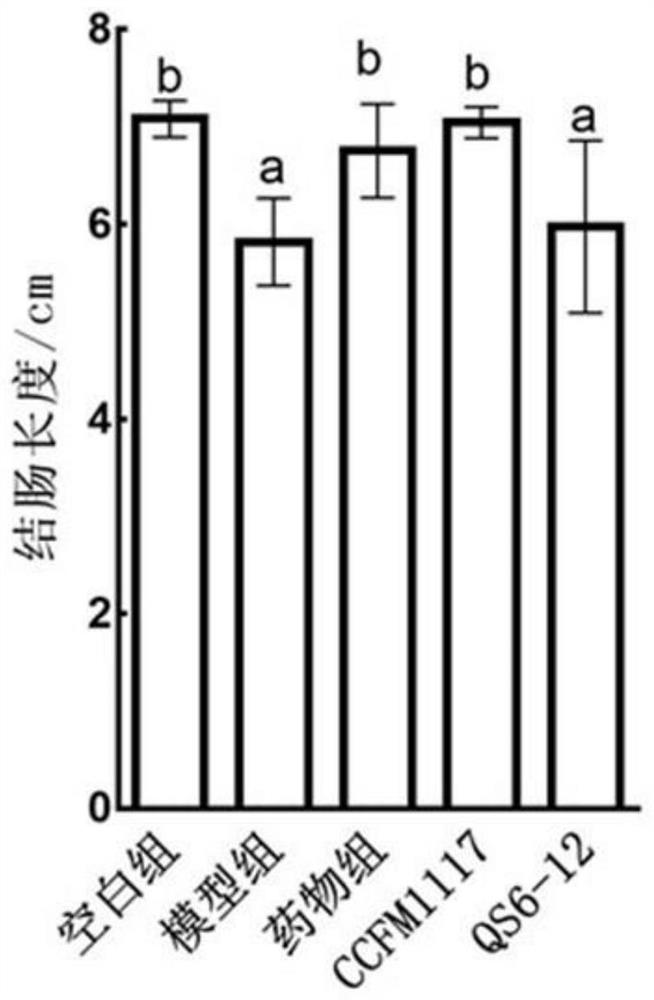
The invention discloses a lactobacillus plantarum for relieving ulcerative colitis and application thereof, and belongs to the technical field of functional microorganisms. The lactobacillus plantarum CCFM1117 can tolerate the gastrointestinal environment of a human body, reduce weight loss in the ulcerative colitis disease period, improve fecal characters and hemafecia conditions, relieve length shortening of the colon, improve colonic mucosal injury, reduce the content of proinflammatory factors TNF-alpha, IL-1beta, IL-6 and IFN-gamma in the colon, up-regulate the gene transcription level of colon tight junction related proteins Claudin-3, ZO-1, ZO-2 and Occludin, increase the content of short-chain fatty acid, increase the abundance of short-chain fatty acid producing bacteria Coprococcus and butyric acid producing bacteria Faecalibacterium, and improve the diversity of intestinal flora.
Owner:JIANGNAN UNIV
Technology for extracting high-purity heparin sodium from intestinal mucosa by trypsin method
The invention relates to the technical field of heparin sodium production, and provides a technology for extracting high-purity heparin sodium from intestinal mucosa by a trypsin method. The technology is characterized by adopting a fresh pig pancreas as the raw material to prepare a trypsin liquid and to extract the heparin sodium, therefore, utilization of artificial compositions is reduced greatly, the product nature is improved, the yield is high, and only about 1600 pig small intestines are needed to produce hundred million international units of the crude heparin sodium product; furthermore, as the trypsin liquid is adopted to extract the heparin sodium, peculiar smell in a production process can be reduced to a large extent, and the environmental protection is facilitated. According to the invention, a re-dissolution and re-precipitation step is added, so that the problem that the product purity is lower due to the fact that salt crystal is formed easily by a high-concentration alcohol and an eluent and is mixed in the heparin sodium product. The purity of the heparin sodium product processed by the technology is improved remarkably, and the titer of the heparin sodium is larger than 130 U / mg.
Owner:HANGZHOU LONGYANG BIOTECH
Application of lactobacillus rhamnosus to preventing and relieving ulcerative colitis
ActiveCN112625964AEasily damagedIncrease richnessMilk preparationBacteriaLactobacillus rhamnosusUlcerative colitis
The invention discloses an application of lactobacillus rhamnosus to preventing and relieving ulcerative colitis, and belongs to the technical field of functional microorganisms. The lactobacillus rhamnosus can tolerate the gastrointestinal environment of a human body, significantly reduce weight loss during the period of ulcerative colitis, improve fecal traits and hematochezia, improve colon mucosal injury, reduce MPO activity, and reduce the content of proinflammatory factors TNF-alpha, IL-1beta, IL-6 and IFN-gamma in colon. The transcription levels of colon close junction related proteins Claudin-3, ZO-1, ZO-2 and Occludin, antibacterial peptides Reg3g and Reg3b and mucoprotein MUC2 are up-regulated, the abundance of short-chain fatty acid producing bacteria Coprococcus and Faecalibacterium and the content of short-chain fatty acid are improved, the abundance of beneficial bacteria Lactobacillus in intestinal tracts is increased, the abundance of the conditional pathogenic bacteria Acinetobacter is reduced, and the abundance and diversity of intestinal flora are increased.
Owner:JIANGNAN UNIV
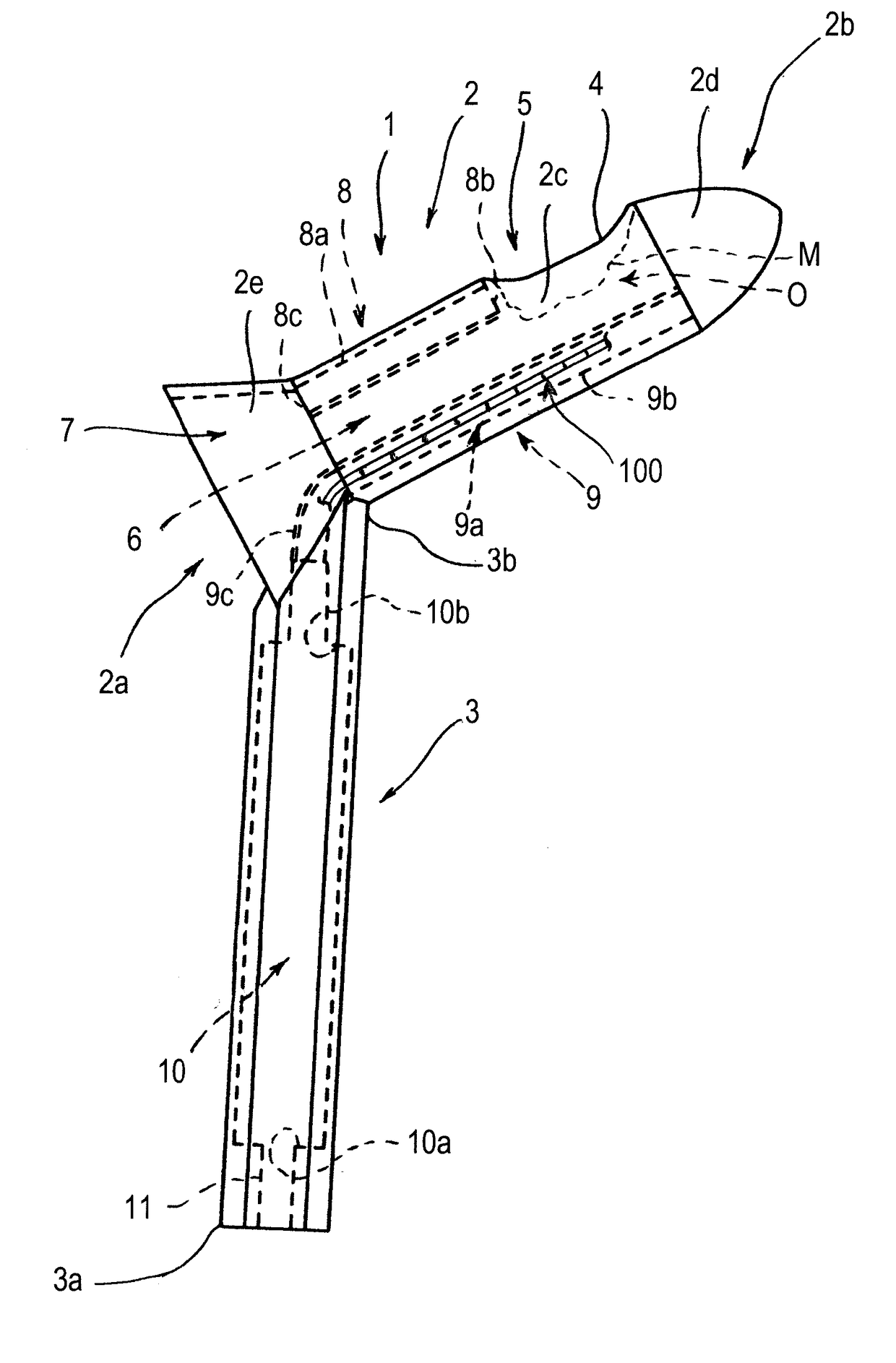
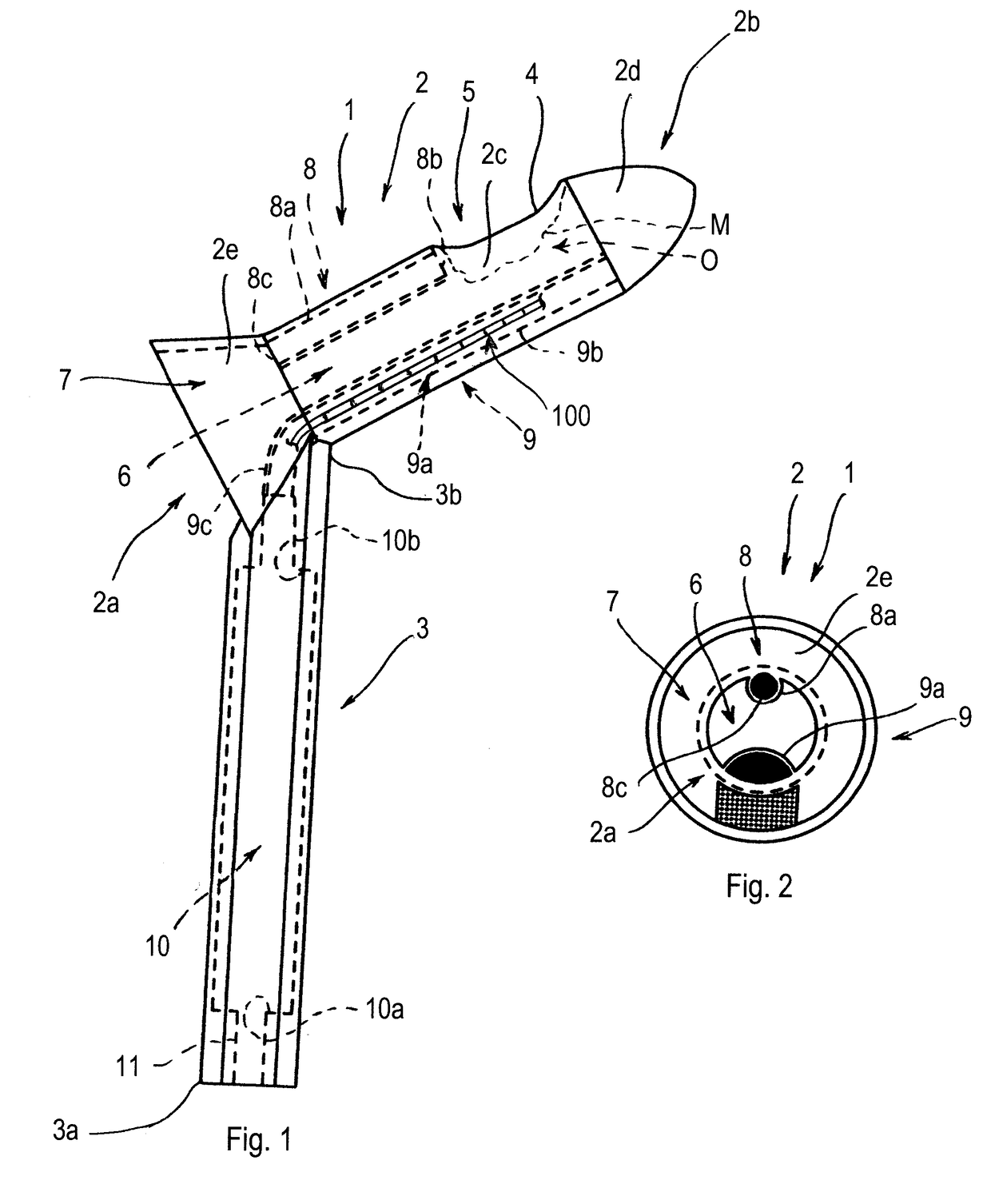
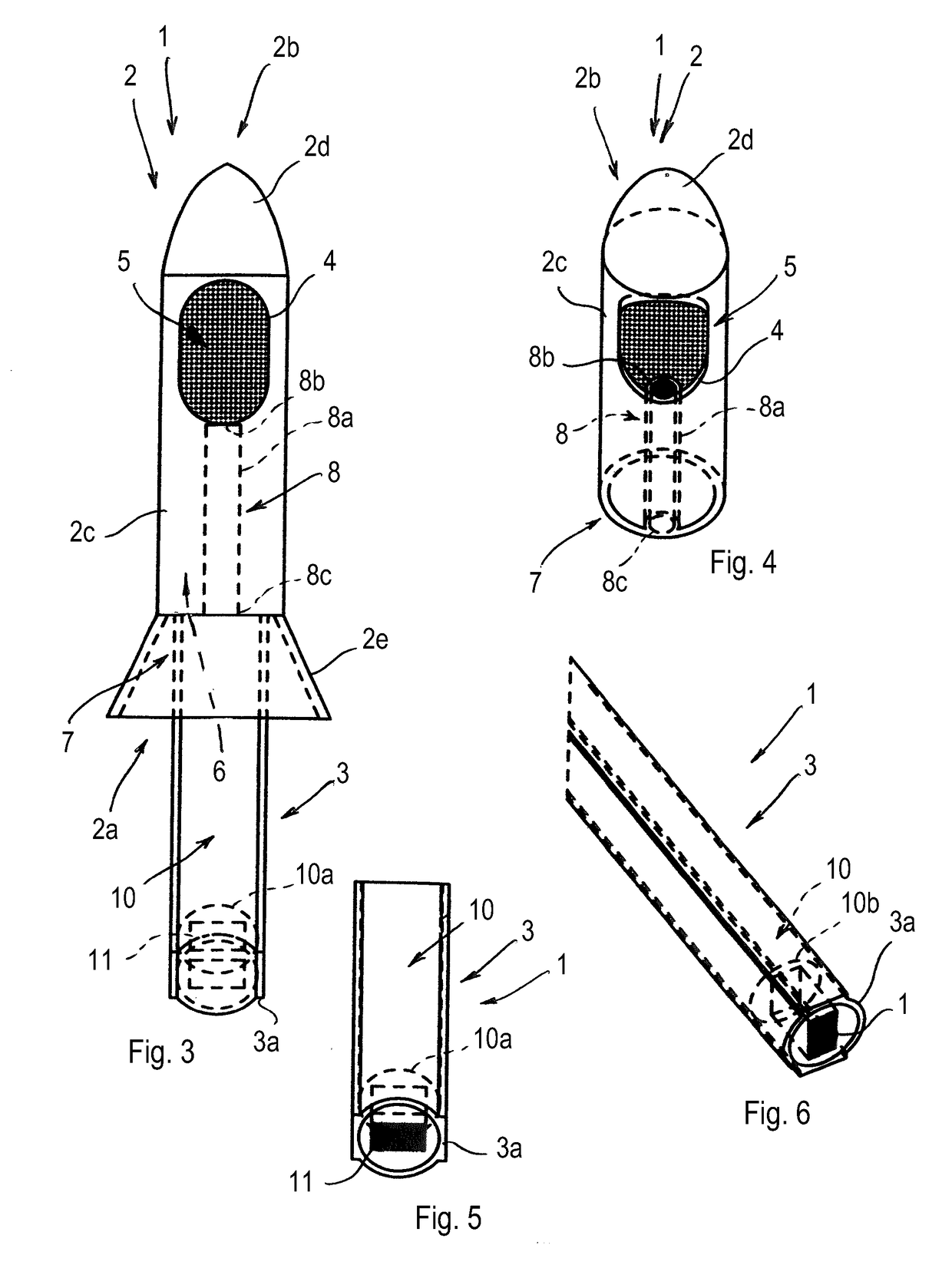
Anoscope
An anoscope comprises a graspable portion, arranged for being grasped by an operator, and a body, arranged for being inserted into the terminal tract of the rectum of a patient. A cavity and at least one operating window are made in the body of the anoscope. The anoscope further comprises a housing and a seat, which are comprised in the cavity of the body. The housing is arranged for containing at least one surgical instrument suitable for treating hemorrhoids and the seat is arranged for containing at least one lighting device. A method for treating a hemorrhoid in a patient comprises the following steps: a) Inserting an anoscope into the terminal tract of the rectum of the patient through the anal opening; b) Positioning the body of the anoscope in such a way that an operating window or opening of the anoscope is near a zone of rectal mucosa to be treated, positioned near the hemorrhoids; c) Positioning a surgical instrument suitable for treating hemorrhoids inside the body of the anoscope so as to reach the operating window or opening and, through the operating window or opening, reaching the zone of rectal mucosa to be treated; d) Producing a localized heat in the submucosa of the zone of rectal mucosa to be treated through the surgical instrument, such localized heat being a heat that is limited to the zone of rectal mucosa to be treated; e) Inducing a volume reduction of the hemorrhoid as a result of the localized heat produced.
Owner:SIAS
Sheep enoxaparin sodium compound preparation method, compound and application of compound
InactiveCN105131153AQuality is easy to controlRaw materials are easy to getOrganic active ingredientsPharmaceutical delivery mechanismSheep farmingMedicine
The invention discloses a method for preparing sheep enoxaparin sodium from sheep intestinal mucosa heparin. The method comprises the following steps: 1, preprocessing sheep heparins; 2, preparing a sheep heparin quaternary ammonium salt; 3, preparing sheep heparin benzyl ester; and 4, carrying out alkali depolymerization on the sheep heparin benzyl ester, decoloring, neutralizing by using an acid, carrying out alcohol precipitation, refining, and drying to obtain finished sheep enoxaparin sodium. The simple and efficient method for preparing the sheep enoxaparin sodium from the sheep intestinal mucosa heparin is screened and established, and researches of the systemic physical and chemical properties, the biological activity and the molecule structure are carried out on the prepared sheep enoxaparin sodium. The sheep enoxaparin sodium prepared in the invention completely accords with USP37 and EP8.0 quality release criteria of sheep enoxaparin sodium, and has extremely high practical values and medical application prospect. The sheep enoxaparin sodium has the advantages of simple and easily available raw material, controllable quality, no existence of bovine spongiform encephalopathy virus risk, promotion of the effective utilization of sheep culture and slaughter wastes (intestinal mucosa), and great economy potential.
Owner:SUZHOU RONGXI BIOTECH CO LTD
Mucosal membrane receptor and uses thereof
ActiveUS20110129525A1Beneficial effectPowder deliveryCompound screeningBacterial diarrheaReceptor for activated C kinase 1
The invention is based on the identification of aminopeptidase N (APN) as the receptor for F4 fimbriae of enterotoxigenic E. coli (ETEC). Based on the observation that oral administration of F4 fimbriae induces a protective intestinal mucosal immune response against a subsequent challenge with F4 ETEC, and the observation that the internalization of said F4 fimbriae is clathrin-mediated, the present invention provides the characterization of APN as a target useful in: in an in vitro assay to screen for molecules that are capable to mimic the clathrin-mediated F4 endocytosis; in an in vitro assay to screen for molecules that are capable to modulate the binding of F4 fimbriae with APN; in the development of a carrier for the delivery of antigens / therapeutics, i.e. immunomodulators to the intestinal submucosa or the intestinal mucosa-associated lymphoid tissue, wherein said carrier comprises an APN specific target molecule that mimics the clathrin-mediated F4 endocytosis. The use of the carriers thus identified or the treatments thus identified, in a method of inducing an antigen specific intestinal mucosal immune response, and / or in the treatment of bacterial diarrhea, is a further aspect of the present invention.
Owner:UNIV GENT
Bifidobacterium longum subsp. Longum capable of preventing and relieving colitis symptoms and application thereof
ActiveCN114164134AEasily damagedEasy to changeBacteriaDigestive systemBiotechnologyUlcerative colitis
The invention provides bifidobacterium longum subsp. Longum KLDS K5 capable of preventing and relieving symptoms of colitis, and belongs to the technical field of microorganisms. The invention further provides a preparation method of the bifidobacterium longum subsp. Longum KLDS K5. Experiments show that the bifidobacterium longum provided by the invention can tolerate the human gastrointestinal environment, relieve weight loss in the ulcerative colitis disease period, improve colonic mucosa injury and reduce MPO activity, and inhibit oxidation-related factors through iNOS and COX-2 signal pathways so as to relieve inflammatory bowel diseases; nF-kappa B p65 nuclear transfer is inhibited through a TLR4 / MyD88 / NF-kappa B signal channel, the expression quantity of proinflammatory factors TNF-alpha, IL-1beta, IL-6 and IL-10 in colon is reduced, the transcriptional level of colon tight connection related proteins Claudin-1, ZO-1 and Occludin and mucoprotein MUC2 is up-regulated, the intestinal flora change after DSS induction can be improved in the genus level, and the richness and diversity of the intestinal flora are improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Technology for extracting heparin sodium from intestinal mucosa with trypsin method
The invention relates to the technical field of heparin sodium production, and provides a technology for extracting heparin sodium from intestinal mucosa with a trypsin method. The technology is characterized by adopting a fresh pig pancreas as the raw material to prepare a trypsin liquid and to extract the heparin sodium, therefore, utilization of artificial compositions is reduced greatly, and the product nature is improved; furthermore, as the trypsin liquid is adopted to extract the heparin sodium, peculiar smell in a production process can be reduced to a large extent, and the environmental protection is facilitated. According to the invention, the fresh pig pancreas is adopted as the raw material to prepare the trypsin liquid and to extract the heparin sodium, the yield is high, only about 1600 pig small intestines are needed to produce hundred million international units of the crude heparin sodium product, the product purity is high, and the titer of the heparin sodium is larger than 100 U / mg.
Owner:HANGZHOU LONGYANG BIOTECH
Bifidobacterium lactis MN-Gup dairy product and application thereof in improving type 2 diabetes mellitus
ActiveCN113207961AIncrease diversityImprove balanceMilk preparationAgainst vector-borne diseasesPancreatic hormoneGut flora
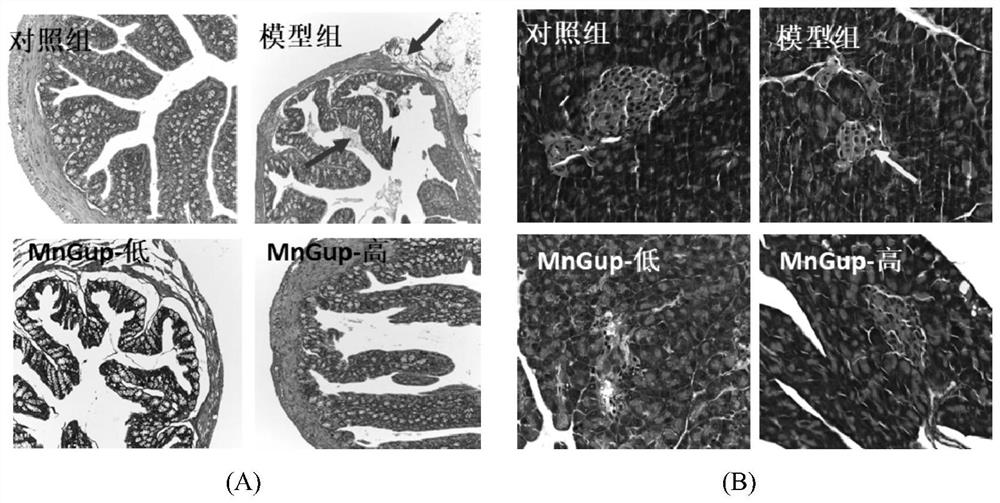
The invention provides a dairy product capable of improving type 2 diabetes mellitus or regulating intestinal flora. The dairy product contains bifidobacterium lactis MN-Gup. The dairy product provided by the invention can improve the characteristic intestinal flora of the type 2 diabetes mellitus in a targeted manner, and regulate the diversity of the intestinal flora and / or the balance of the intestinal flora; the dairy product can improve the type 2 diabetes mellitus through a comprehensive mechanism, including significant relieving of weight loss and islet injury, blood sugar level reduction, intestinal mucosa repairing, intestinal insulin improvement, in-vivo inflammation state improvement and healthy intestinal flora recovery.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Probiotic reinforced microelement-vitamin solid beverage and preparation method thereof
ActiveCN110063445AReduce moisture contentGreat tasteMilk preparationVitamin food ingredientsBiotechnologyNutrition
The invention discloses a probiotic reinforced microelement-vitamin solid beverage and a preparation method thereof. (Live) Multi-strain probiotics which are embedded by three layers and can colonizeon intestinal mucosa through a gastric acid barrier are applied, a nutrient supplement, multivitamins, mineral elements are used as main materials, and the ingredients homologous in medicine and food:alpha-linolenic acid, black sesame, brown sugar and other auxiliary materials are used as carriers. The preparation method comprises the steps: mixing and stirring the main materials and the auxiliary materials for multiple times, conveying the evenly stirred materials at 40-46 DEG C to a chocolate coater inlet through a closed conveying belt for twice chocolate coating. Under the molding processcondition, the air moisture content is the lowest, the probiotics are prevented from being inactivated, the shapes of pressed blocks are stabilized through chocolate spraying, the products are protected from light and oxidation, and the total water content of the probiotic solid beverage is effectively controlled to 7% or below, and the probiotic reinforced microelement-vitamin solid beverage cansmoothly pass through the gastric acid barrier, can be planted on intestinal mucosa for self-propagation and amplification, and is a new product which is convenient to store, carry and eat, good in taste, rich in nutrition and high in cost performance.
Owner:杨成林
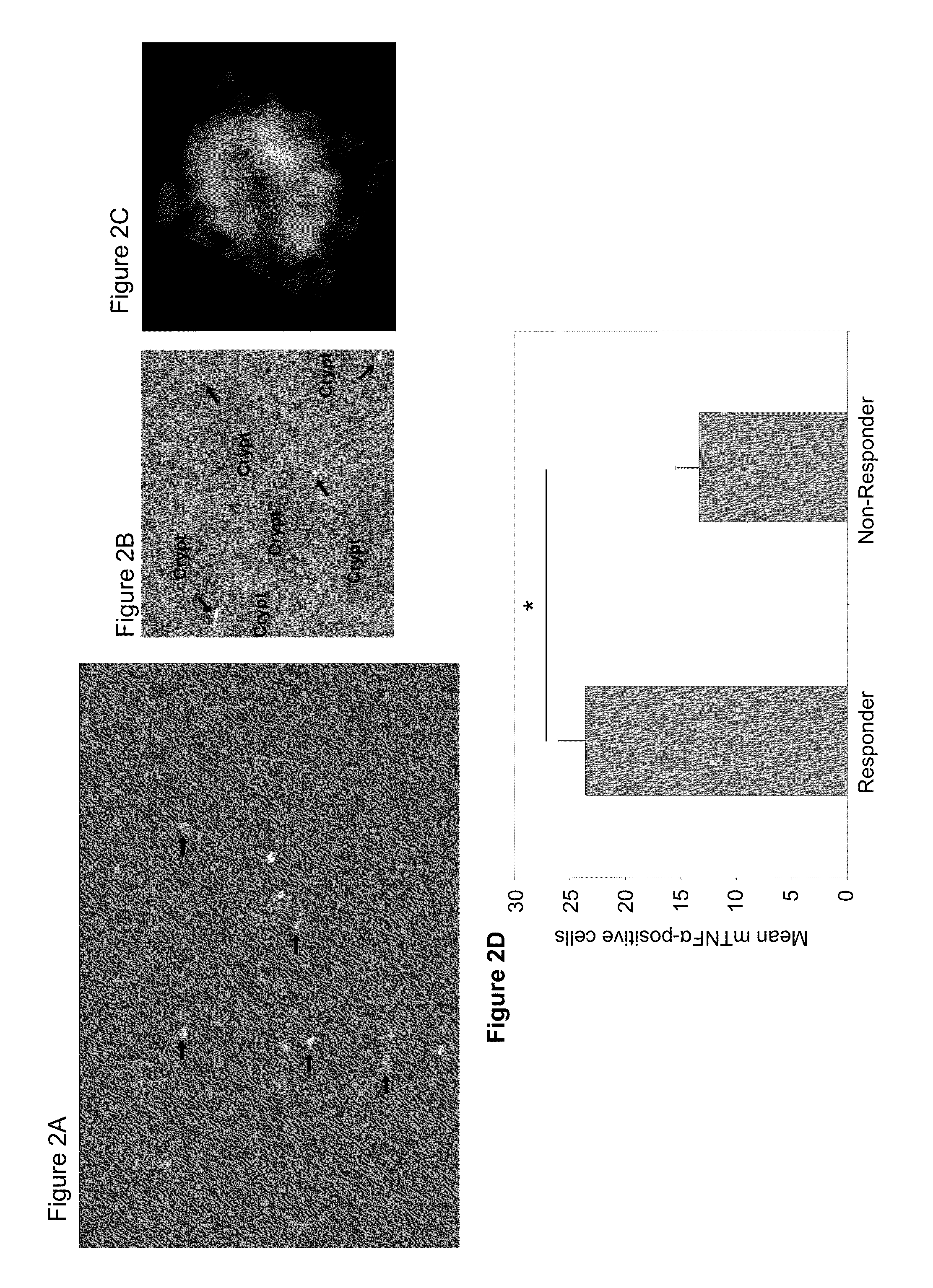
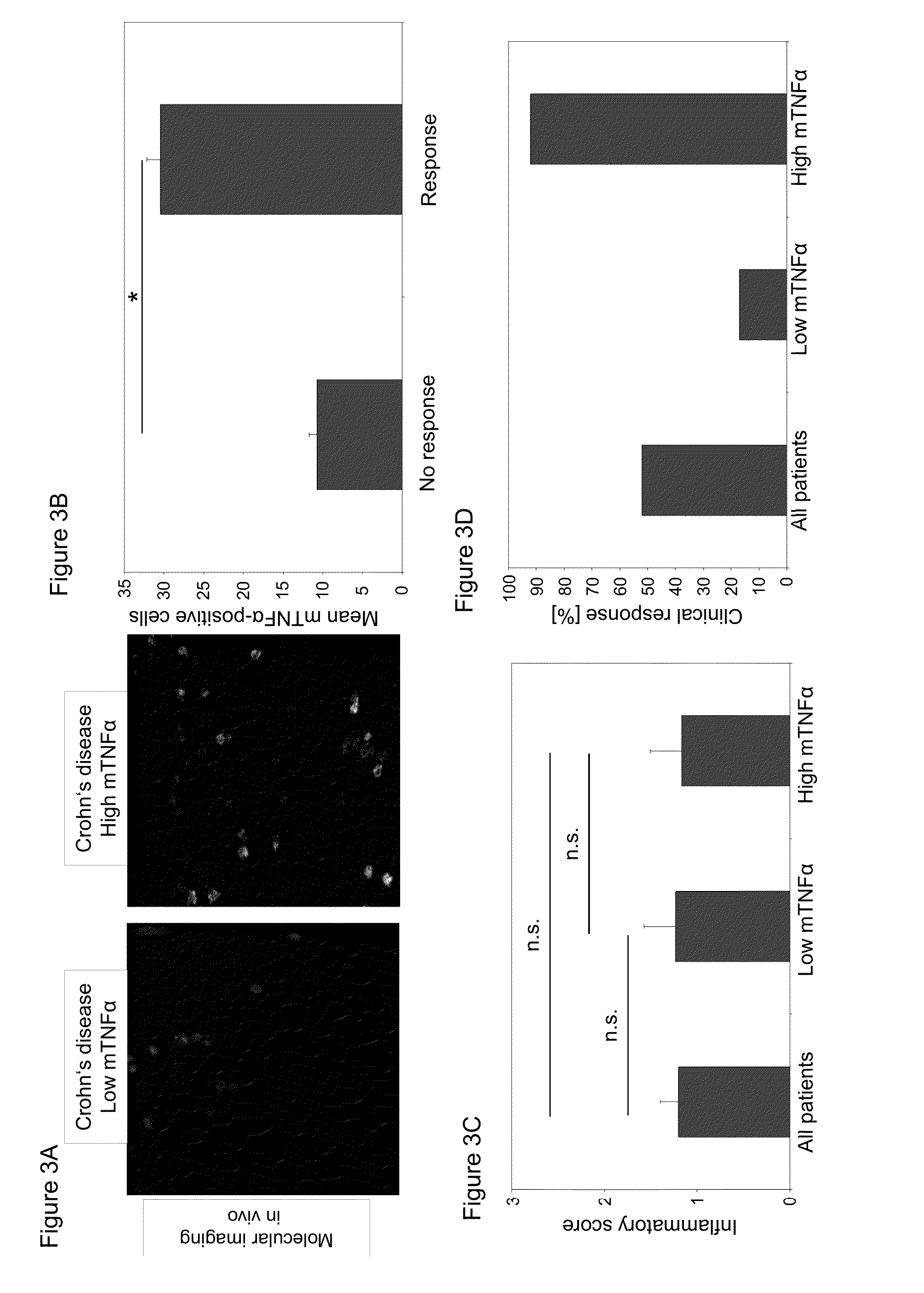
Methods and compositions for determining responsiveness to treatment with a tnf-alpha inhibitor
InactiveUS20140017174A1Safe and effective method of treatingReduced systemic exposureUltrasonic/sonic/infrasonic diagnosticsLibrary screeningInflammatory Bowel DiseasesParentucellia
The present invention is directed to methods and compositions useful for predicting the efficacy of a TNFα inhibitor for treating an inflammatory bowel disease (IBD). The invention includes, in one embodiment, determining the level of expression of TNFα by delivering a labeled anti-TNFα antibody on to the cells of the intestinal mucosa of a subject having IBD, whereby the TNFα level of expression can be used to predict whether the subject will be responsive or not to the antibody therapy. Levels of TNFα may be determined in vivo or ex vivo. The invention further provides methods of locally administering a TNFα antibody, e.g., topically to the intestinal mucosa, for the treatment of IBD.
Owner:ATREYA RAJA +1
Novel technology for extracting high purity heparin sodium from intestinal mucosa
InactiveCN103130916AControlling Oxidative InactivationHigh recovery rateIntestinal structurePurification methods
The invention relates to a preparation and purification method for biological medicinal raw material, belonging to the field of bioengineering, and in particular relates to a technology for preparing high purity low molecular heparin sodium. The method aims at overcoming the defects of the prior art. The invention provides a convenient technology for preparing and purifying low molecular heparin sodium. The average molecular mass of the prepared low molecular heparin sodium is about 4.5KDa, the titer of the prepared low molecular heparin sodium is not lower than 180U / mg and is higher than 170U / mg required in Chinese pharmacopoeia, and the prepared low molecular heparin sodium is up to standard according to the test standard of the heparin sodium in the Chinese pharmacopoeia.
Owner:RUGAO YONGXING CASING
Bifidobacterium lactis MN-Gup and application of microbial inoculum thereof in treatment of type 2 diabetes mellitus
ActiveCN113197921ADamage reliefHigh expressionMetabolism disorderDigestive systemBifidobacteriumMedicine
The invention belongs to the technical field, and particularly relates to application of bifidobacterium MN-Gup and a microbial inoculum thereof in treating type 2 diabetes mellitus. The invention provides bifidobacterium lactis MN-Gup or a microbial inoculum with a main component of bifidobacterium lactis MN-Gup, which can improve type 2 diabetes mellitus through a comprehensive mechanism, such as relieving islet injury, repairing intestinal mucosa, improving related inflammatory response, improving expression of intestinal insulin GLP-1, recovering intestinal flora health and the like. The problem that at present, a probiotic for specifically adjusting type 2 diabetes related intestinal flora and recovering healthy intestinal flora to improve, relieve or treat type 2 diabetes is lacked is solved.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Compound probiotics preparation with gastric and intestinal mucosa protection effect, and application of compound probiotics preparation
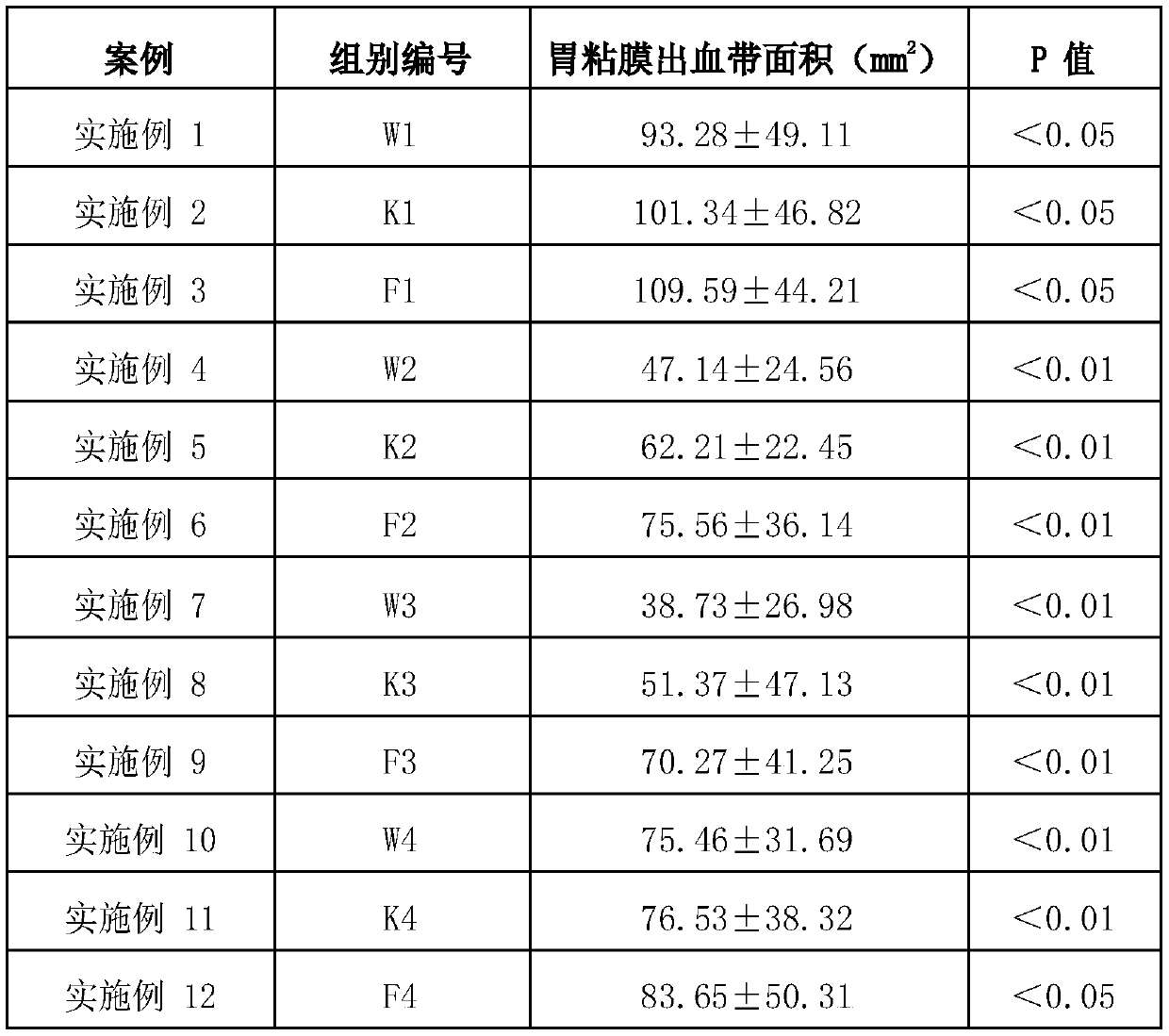
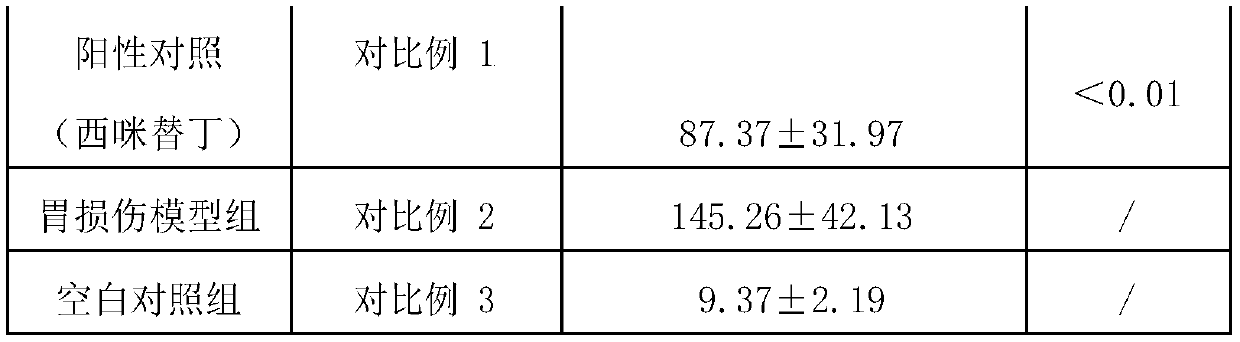
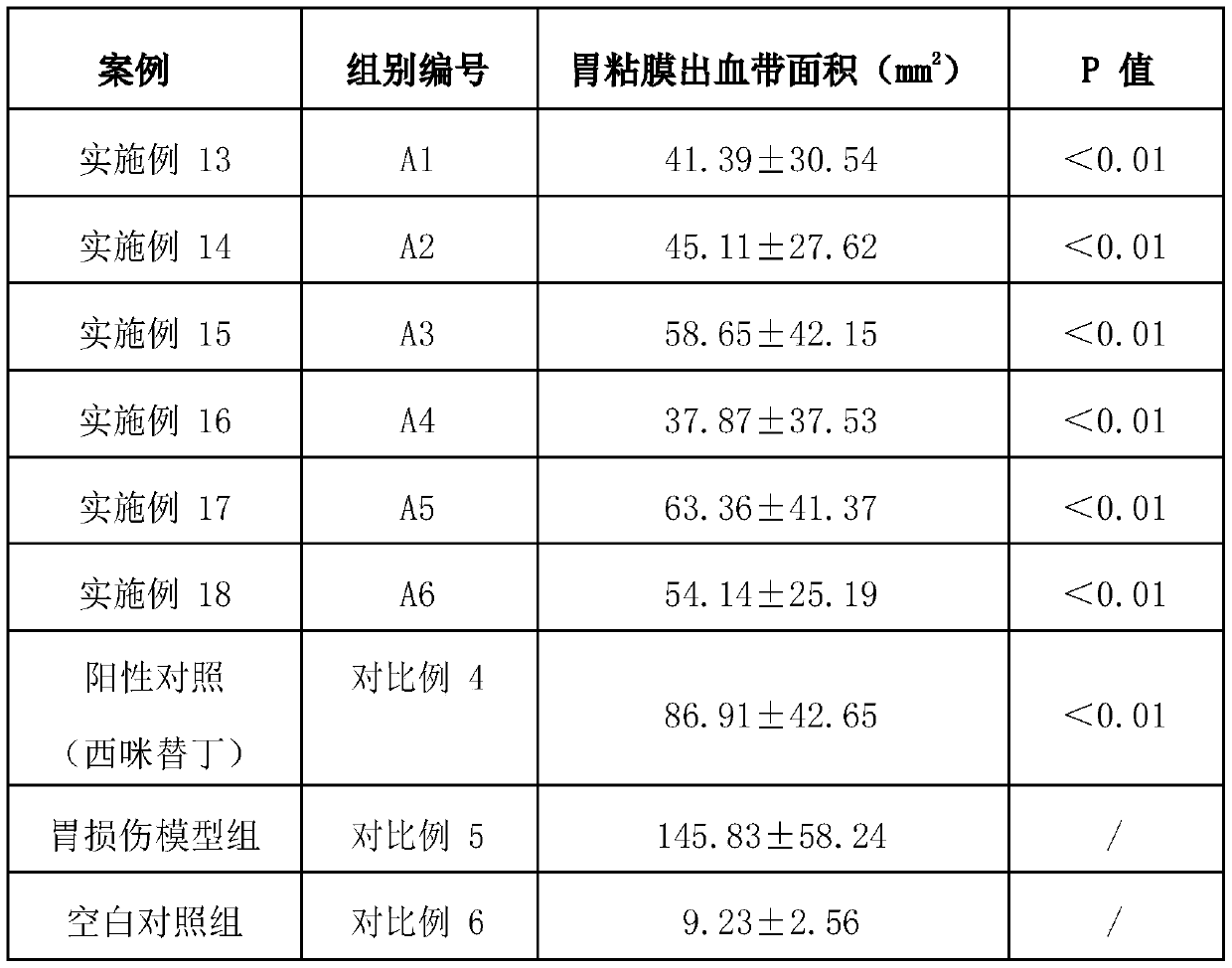
The invention relates to the technical field of microorganisms, and discloses a compound bacterium agent. The compound bacterium agent is prepared from at least two of bifidobacterium longum, lactobacillus paracasei and lactobacillus plantarum. Compared with a model control group, through the compound bacterium agent, the rat gastric mucosa bleeding belt area can be significantly decreased, the gastric mucosa blood flow is increased, and the gastric mucosa damage index is decreased. The mouse colon length is significantly increased, the mouse colon tissue pathology score is decreased, and ulcerative colitis caused by intestinal mucosa damage of mice is relieved. Therefore, the probiotics compound bacterium agent has a certain gastric and intestinal mucosa protection effect, and is very suitable for preparation of food, health care products, medicines and food supplements.
Owner:GUANGDONG YIKEWEI BIOLOGICAL TECH CO LTD
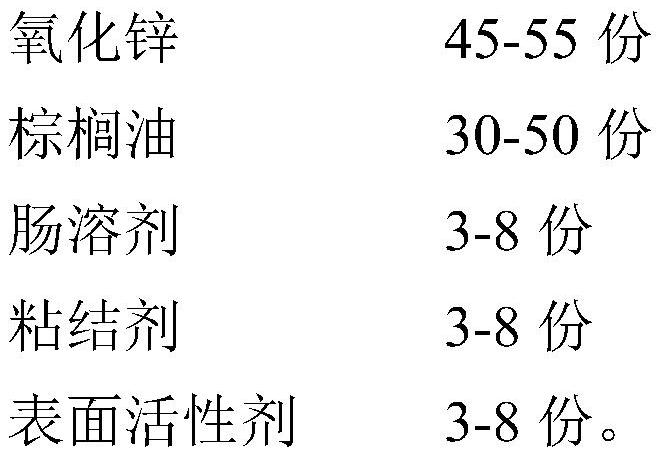
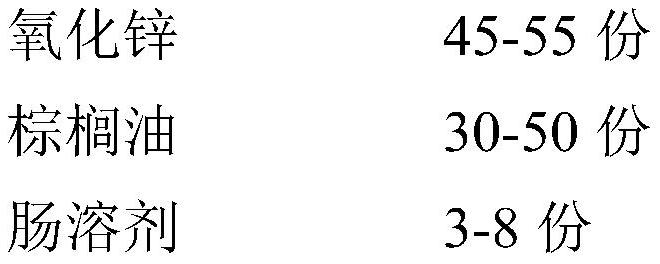
Enteric coated nano zinc oxide particles and production method thereof
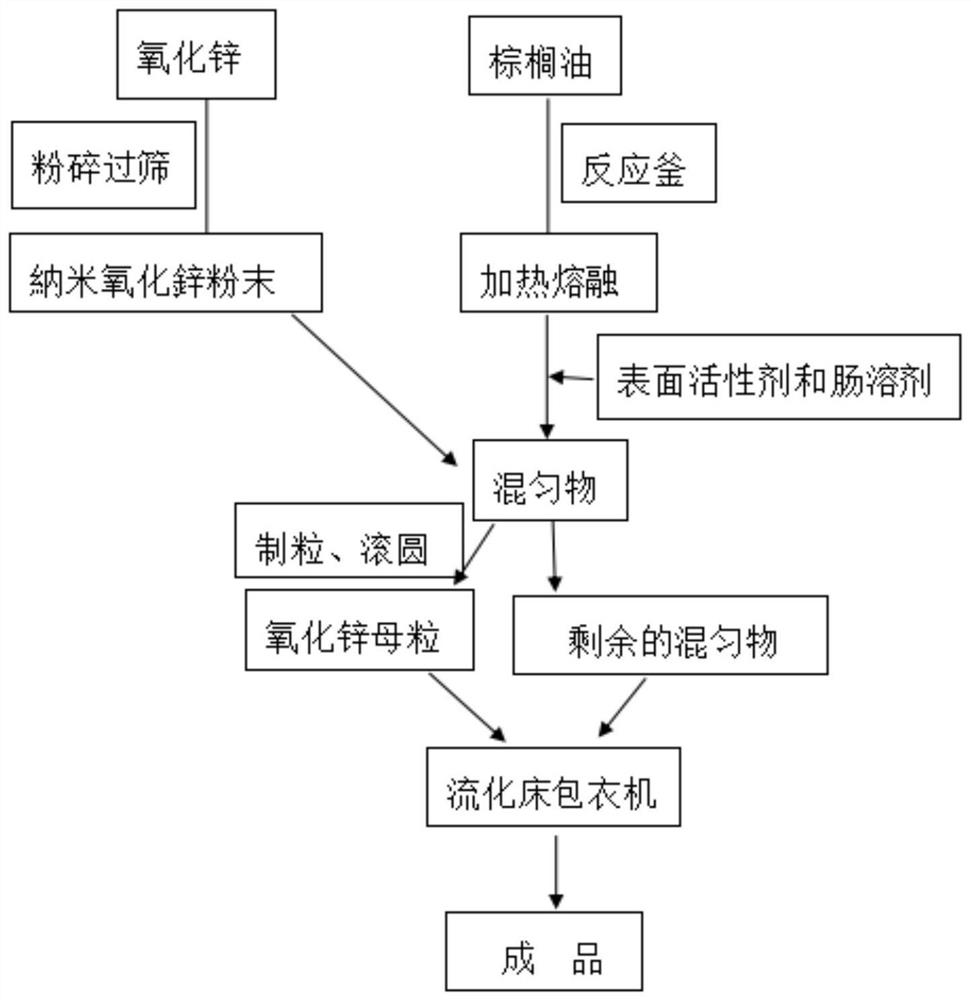
PendingCN112568328AGood dispersionIncrease dissolution rateAccessory food factorsWorking-up animal fodderBiotechnologyActive agent
The invention provides a production method of enteric coated nano zinc oxide particles, and belongs to the technical field of feeds and feed additives. The production method comprises the following process steps: firstly sieving a zinc oxide raw material, adding auxiliary materials, granulating, rounding and drying, heating palm oil to melt, adding a surfactant, spraying the surfactant into a dried material in a fluidized bed, coating, sieving after coating is finished, and sieving to obtain the enteric coated zinc oxide particle product. The zinc oxide particles prepared by the method disclosed by the invention ensure that zinc oxide is not destroyed by gastric acid in the stomach, is disintegrated and released after reaching the intestinal tract, and is combined with the intestinal mucosa, so that the convergence and bacteriostasis effects of zinc oxide are effectively enhanced, and the immunity of animal organisms is improved.
Owner:WUXI ZHENGDA POULTRY
Heparin sodium balance extraction method
The invention discloses a heparin sodium extraction method. The heparin sodium extraction method is characterized in that the method solves problems of insufficient intestinal mucosa enzymatic hydrolysis and bad adsorption effect of traditional enzymatic hydrolysis technologies. A non-uniform enzymatic hydrolysis phenomenon is generated in the traditional enzymatic hydrolysis technologies. The method enables full enzymatic hydrolysis to be carried out through introducing different supersonic waves in different enzymatic hydrolysis phases, so the content of heparin sodium in the obtained enzymatic hydrolysis liquid is improved, and the heparin sodium is fully released. The method enables an adsorption mother liquid to be purified through filtering in an adsorption phase, so the adsorption environment is clean, the adsorption of the resin is easy, and pollution and obstruction are less, thereby the production efficiency of the heparin sodium is improved. The heparin sodium yield is improved by 10-20% through the above two steps, so the method has a substantial economic benefit.
Owner:谭科
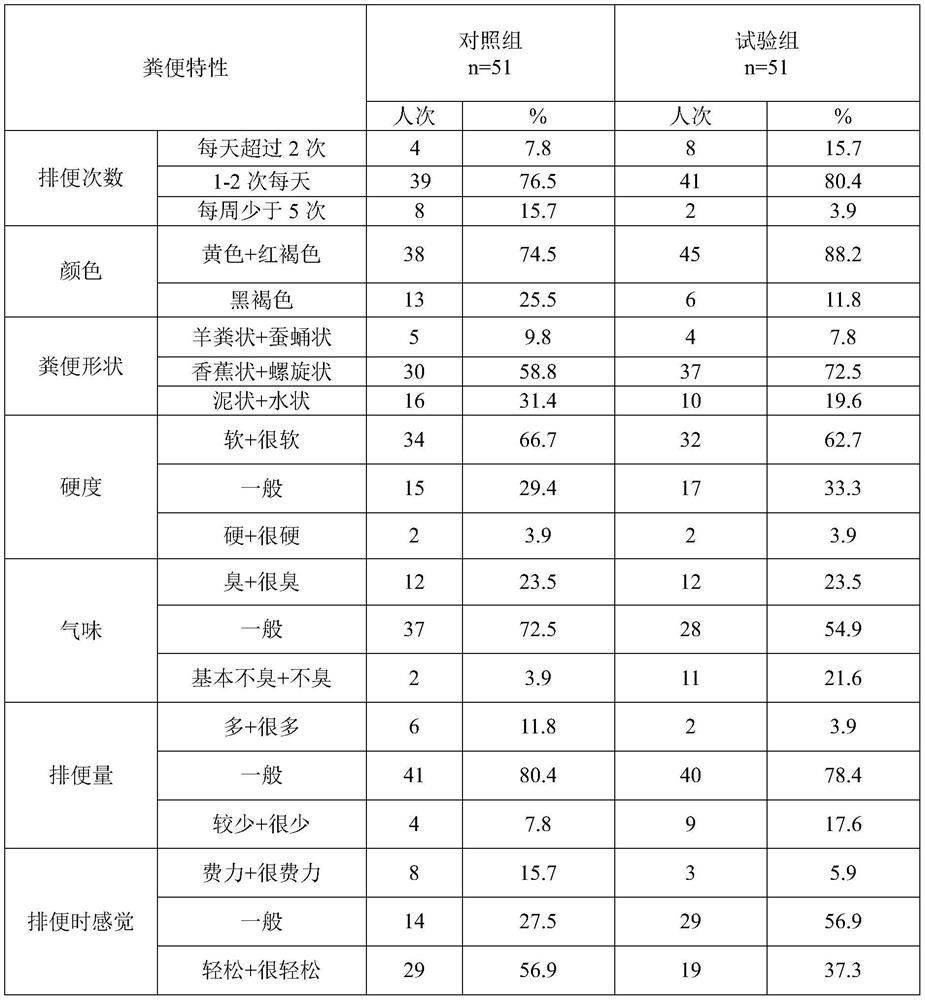
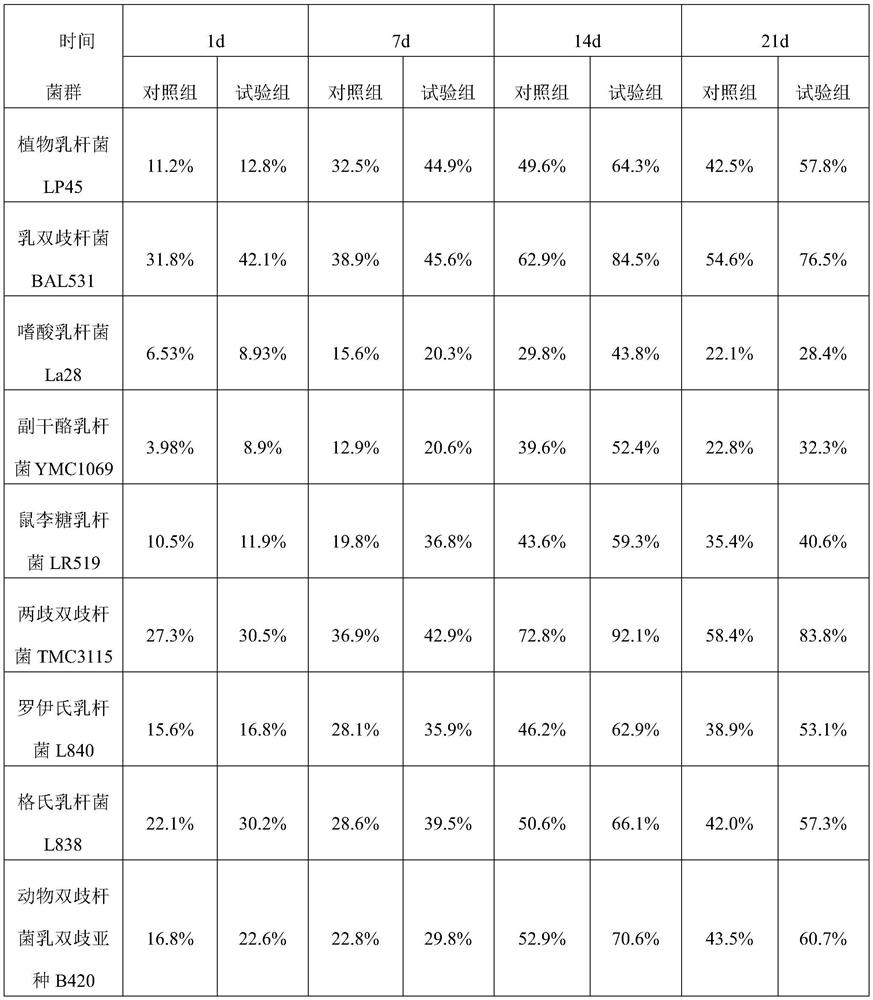
Method for improving survival rate and planting rate of probiotic in intestinal tract
InactiveCN112293731ALong-term colonizationLong-term reproductionMilk preparationFood ingredient functionsBiotechnologyIntestino-intestinal
The invention provides probiotic. The probiotic comprises a bacteria strip and a light fluid, wherein the bacteria strip comprises prebiotics, a strawberry fruit powder, lactobacillus plantarum LP45,bifidobacterium lactis BAL531, lactobacillus acidophilus La28, lactobacillus paracasei YMC1069, lactobacillus rhamnosus LR519, bifidobacterium bifidum TMC3115, lactobacillus reuteri L840, lactobacillus gasseri L838 and bifidobacterium animalis subsp. Lactis B420; and the light fluid comprises skimmed milk powder, maltodextrin, whey protein concentrate powder, fruit powder, konjaku flour, coconut powder, a sugar substitute, a freeze-dried fruit granule, chia seed, psyllium husk and compound vitamins. The invention also provides a method for improving the survival rate and planting rate of the probiotic in the intestinal tract. According to the method, a manner of first taking the light fluid on an empty stomach and then mixing the light fluid with the bacteria strip and then taking the mixture is adopted, the light fluid which is first taken neutralizes gastric acid and bile salt, then the bacteria strip is fully fused with the light fluid, and then a flora of the probiotic is successfully planted in the intestinal mucosa.
Owner:阎飞
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com