Method for recombinant production of pancreatic glucagons sample peptide-2
A peptide sequence, tandem repeat technology, applied in the field of genetic engineering, can solve the problems of technical difficulty and high drug cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
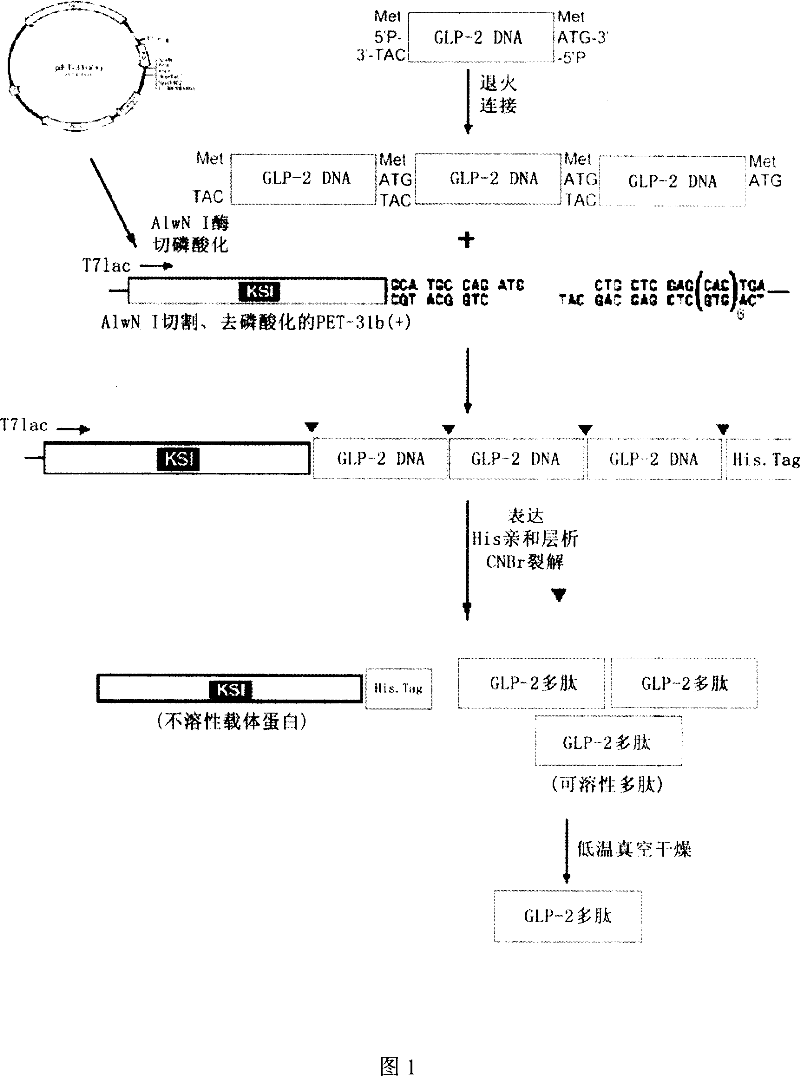
[0054] With the maturity of molecular biology technology, gene recombinant polypeptide is becoming an important way of drug research and development. Compared with peptide synthesis, genetic recombination has the following advantages: 1. High biological activity; 2. Economical, the cost is less than 10% of peptide synthesis; 3. Easy molecular modification to make its effect more obvious. For GLP-2, its molecular weight is only 3.9kDa, so it is difficult to express it by common gene recombination. In a specific embodiment of the present invention, we designed the following method to make expression and purification easier. Its characteristics are:
[0055] 1. Select the pET31 protein expression system that is more suitable for the expression of small molecule polypeptides, and design 3 copies of GLP-2, so that the molecular weight of its expression can reach 24kDa, which is the most suitable molecular weight range for protein expression. 3 copies can make GLP-2 obtain rate is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com