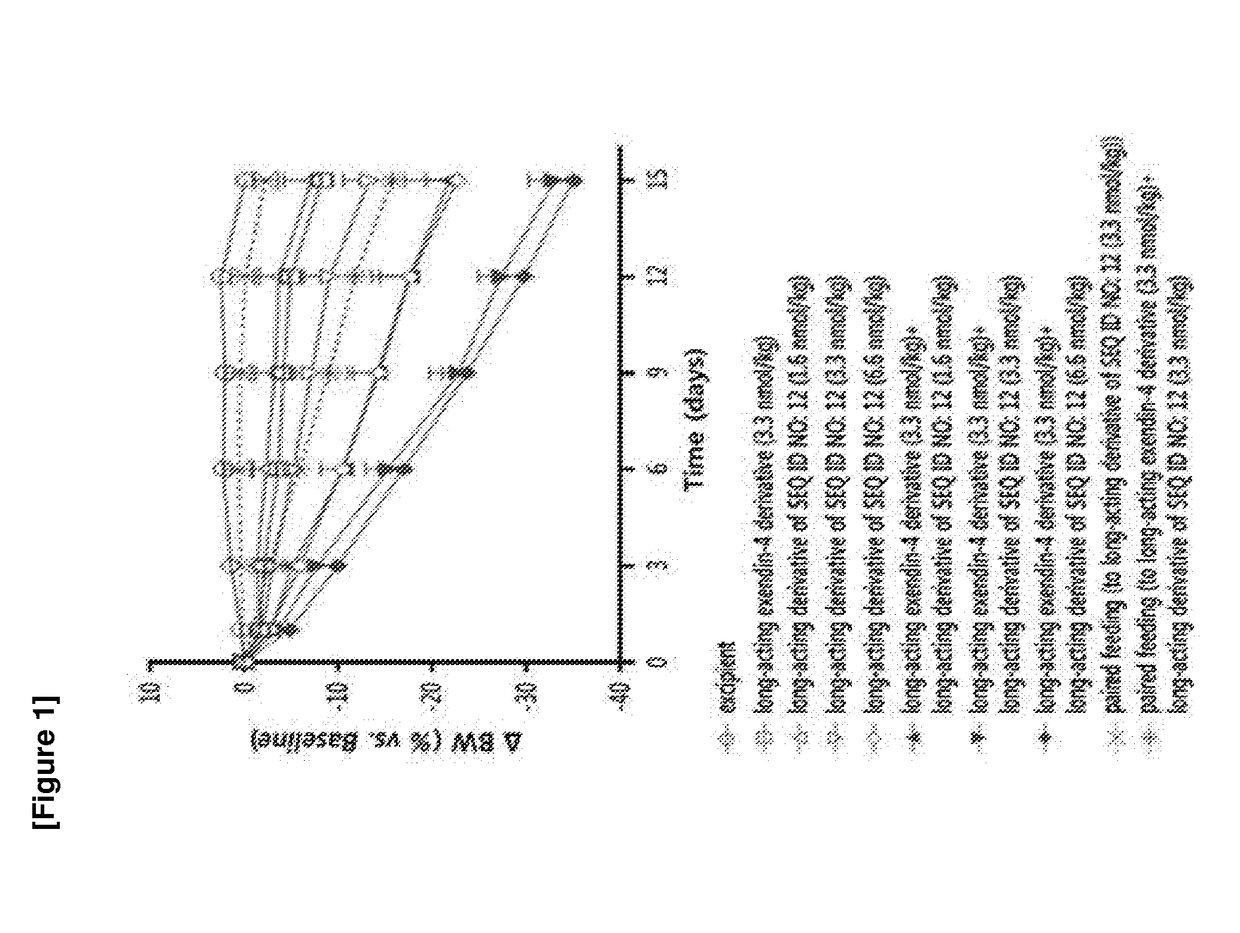
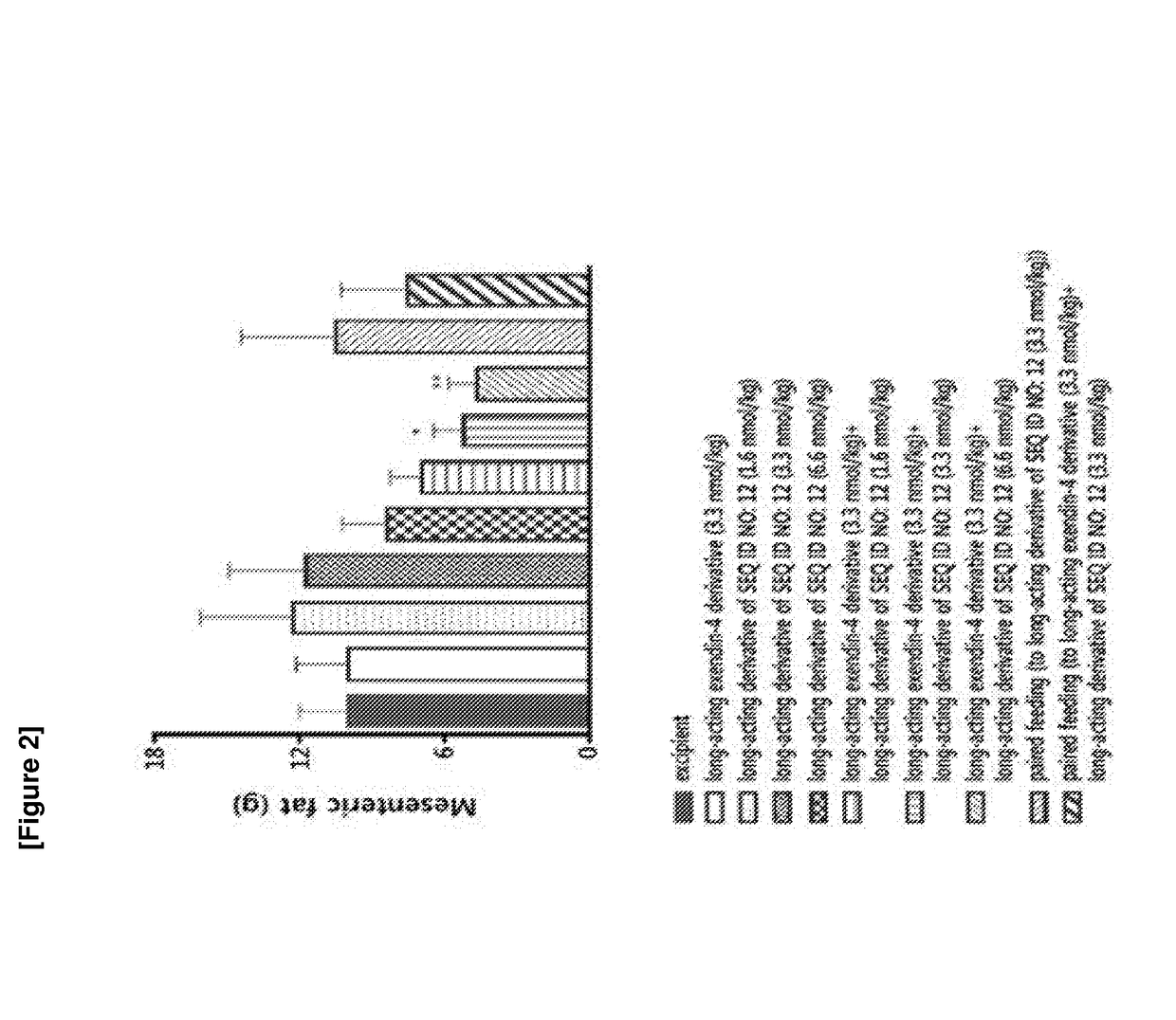
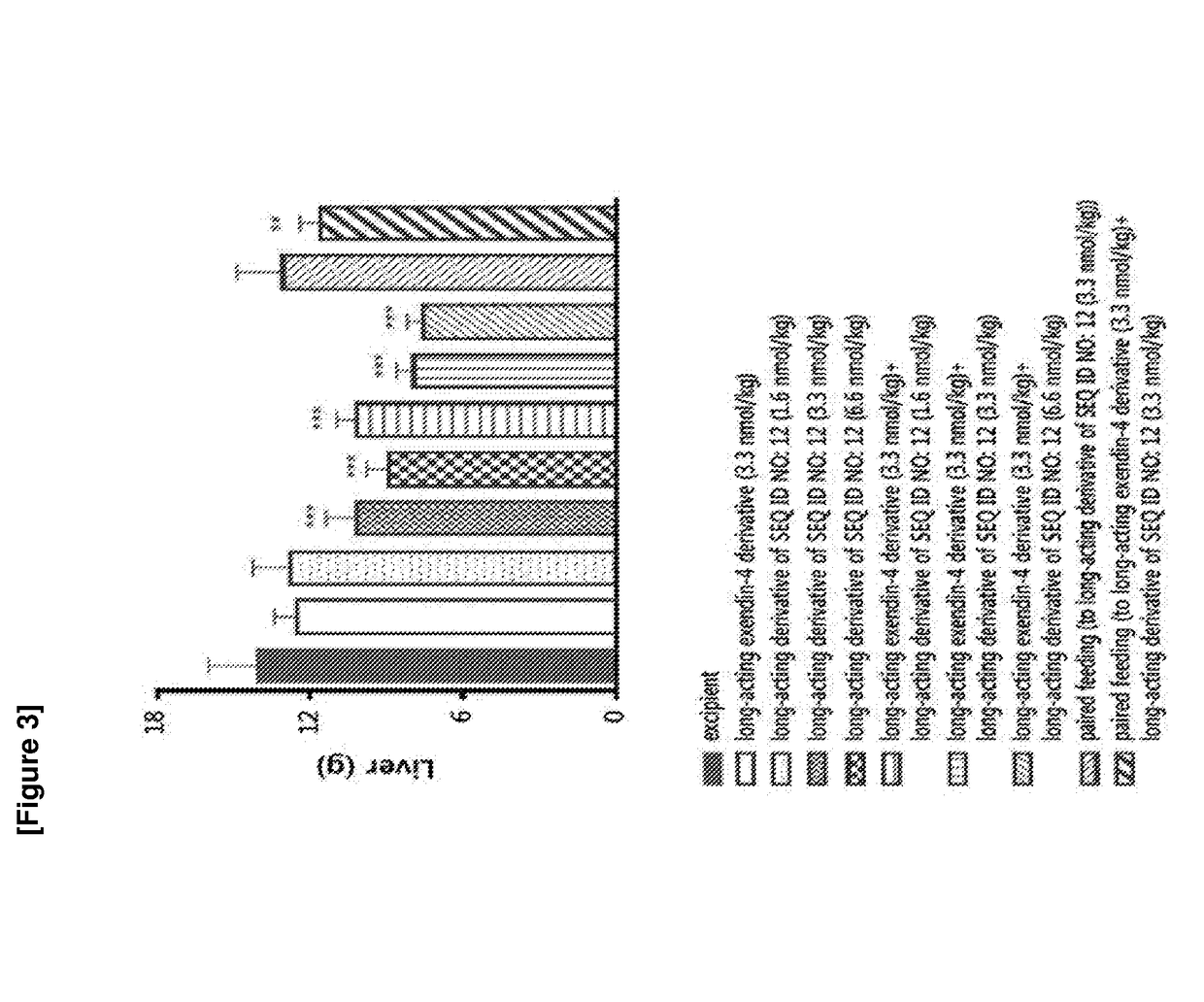
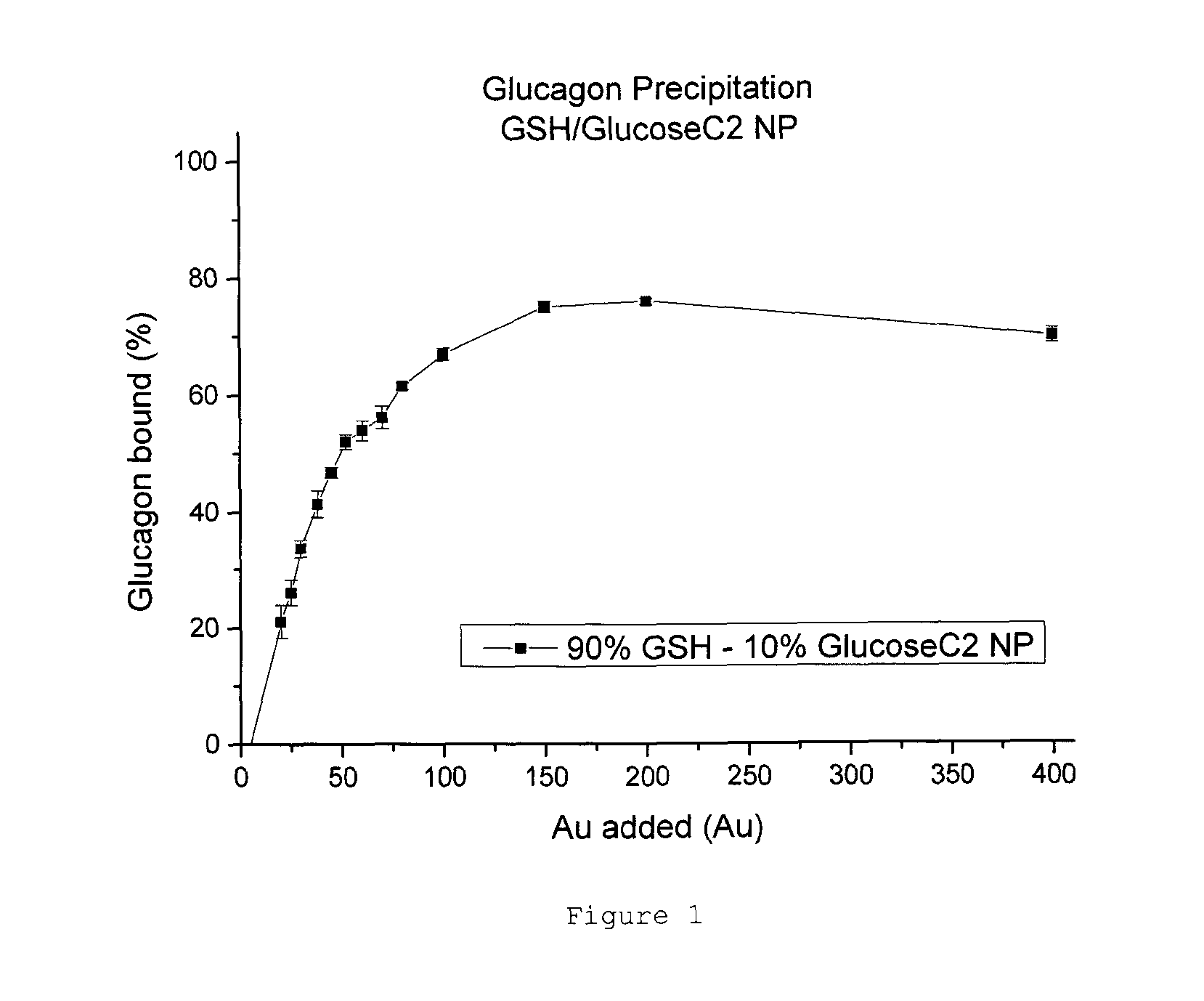
Patents
Literature
50 results about "Pancreatic glucagon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
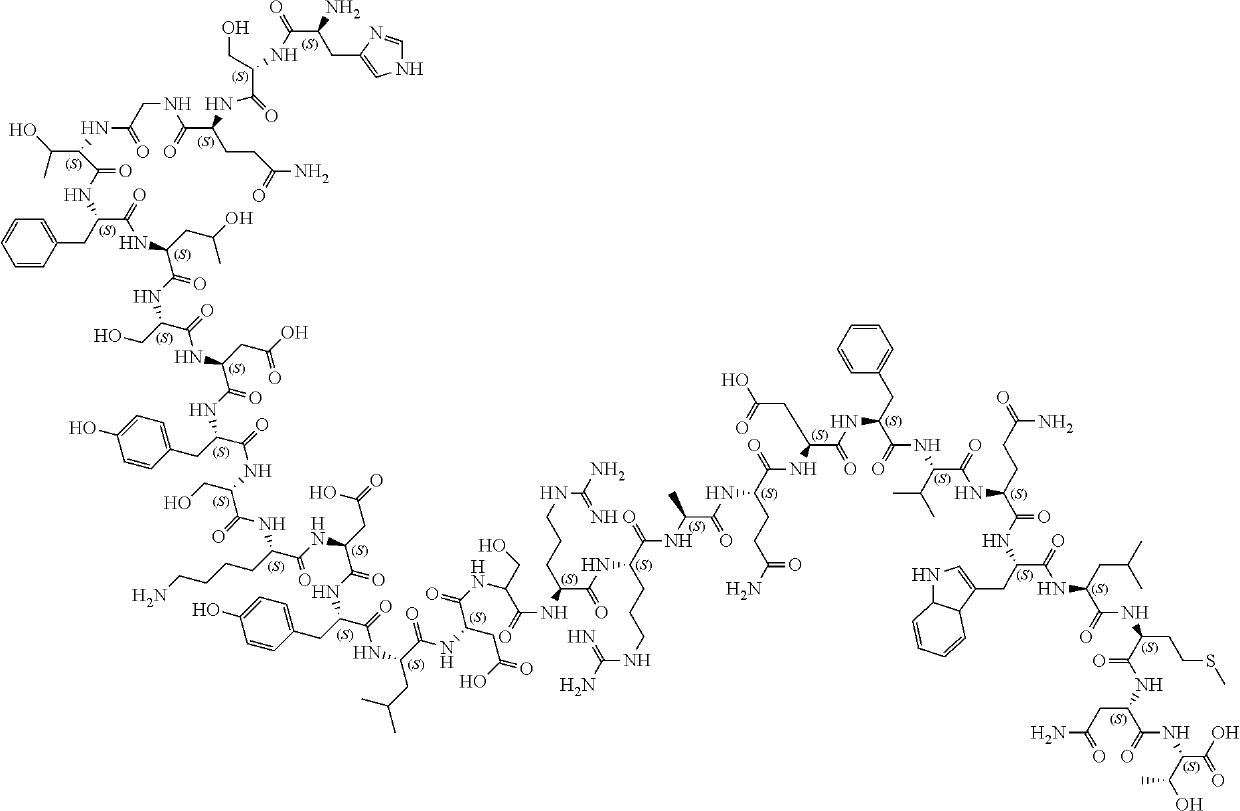
Glucagon, a pancreatic hormone produced by cells in the islets of Langerhans. Glucagon is a 29-amino-acid peptide that is produced specifically by the alpha cells of the islets. It has a high degree of similarity with several glucagon-like peptides that are secreted by cells scattered throughout the gastrointestinal tract.
Glucagon analogues
ActiveUS20140080757A1Avoid weight gainGood for weight lossBacteriaPeptide/protein ingredientsProviding materialObesity
The invention provides materials and methods for the treatment of obesity and excess weight, diabetes, and other associated metabolic disorders. In particular, the invention provides novel glucagon analogue peptides effective in such methods. The peptides may mediate their effect by having increased selectivity for the GLP-1 receptor as compared to human glucagon.
Owner:ZEALAND PHARM AS
Human glucagon-like peptide-1 compound and its preparing method
InactiveCN1676163AImprove biostabilityImprove bioavailabilityPeptide/protein ingredientsMetabolism disorderMonomethoxypolyethylene glycolGlucagon-like peptide-1
The present invention relates to a human pancreatic glucagons sample peptide-1 compound and its preparation method. The human pancreatic glucagons sample peptide-1 in said compound is human pancreatic glucagons sample peptide-1(7-37) or human pancreatic glucagons sample peptide-1(7-36)Nh2. Said compound is formed from human pancreatic glucagons sample peptide-1 and monomethoxy polyglycol containing active group activated by succinimide, namely said compound is mPEG-GLP-1.
Owner:EAST CHINA NORMAL UNIV
Method for recombinant production of pancreatic glucagons sample peptide-2
InactiveCN101041818AIncrease contentBacteriaPeptide/protein ingredientsPancreatic glucagonDrug biological activity
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Glucagon receptor modulators
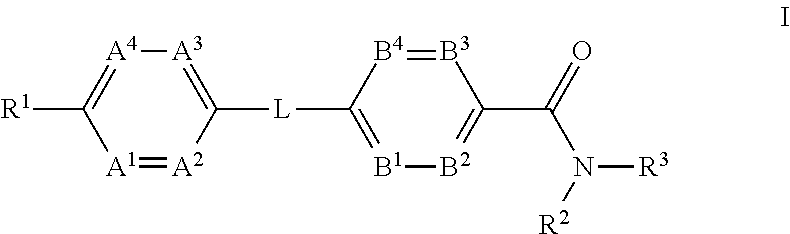
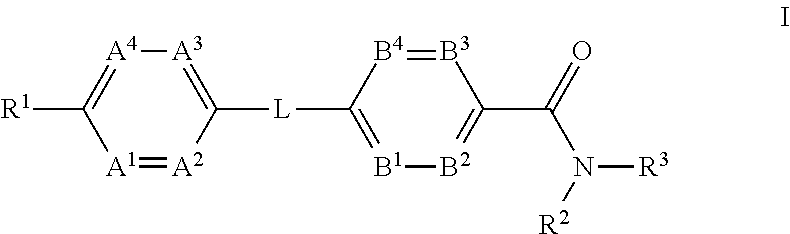
ActiveUS20120202834A1Reduce level of blood glucoseLower blood sugar levelsBiocideOrganic chemistryInverse agonistStereochemistry
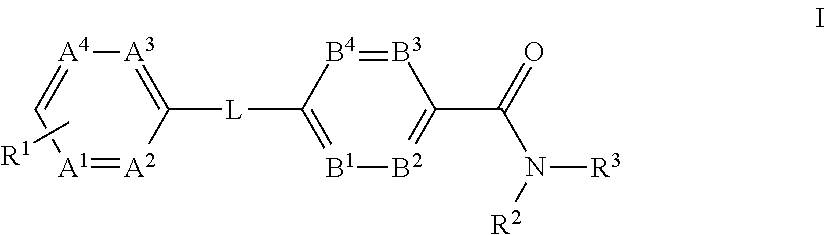
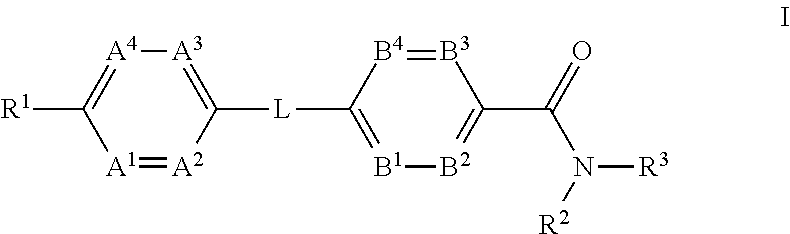
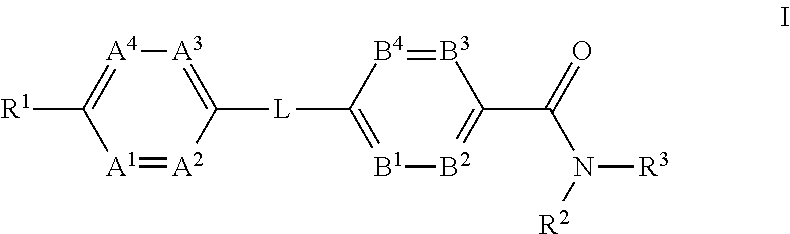
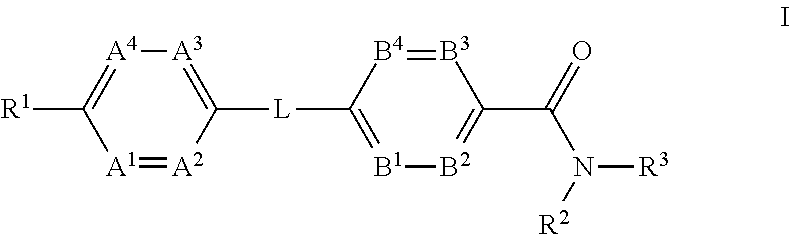
The present invention provides a compound of Formula (I)or a pharmaceutically acceptable salt thereof wherein R1, R2, R3, A1, A2, A3, A4, L, B1, B2, B3 and B4 are as defined herein. The compounds of Formula I have been found to act as glucagon antagonists or inverse agonists. Consequently, the compounds of Formula I and the pharmaceutical compositions thereof are useful for the treatment of diseases, disorders, or conditions mediated by glucagon.
Owner:PFIZER INC
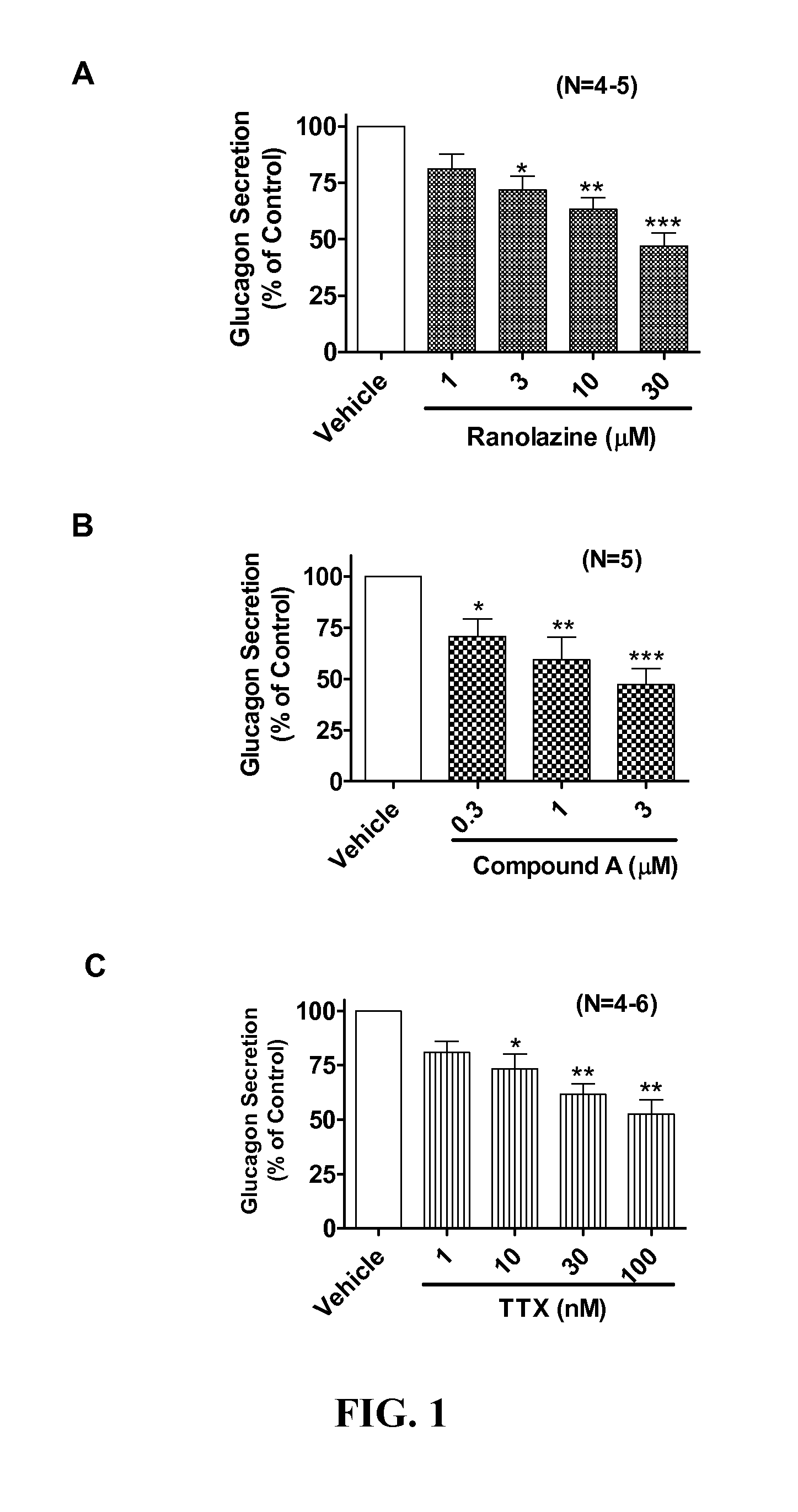
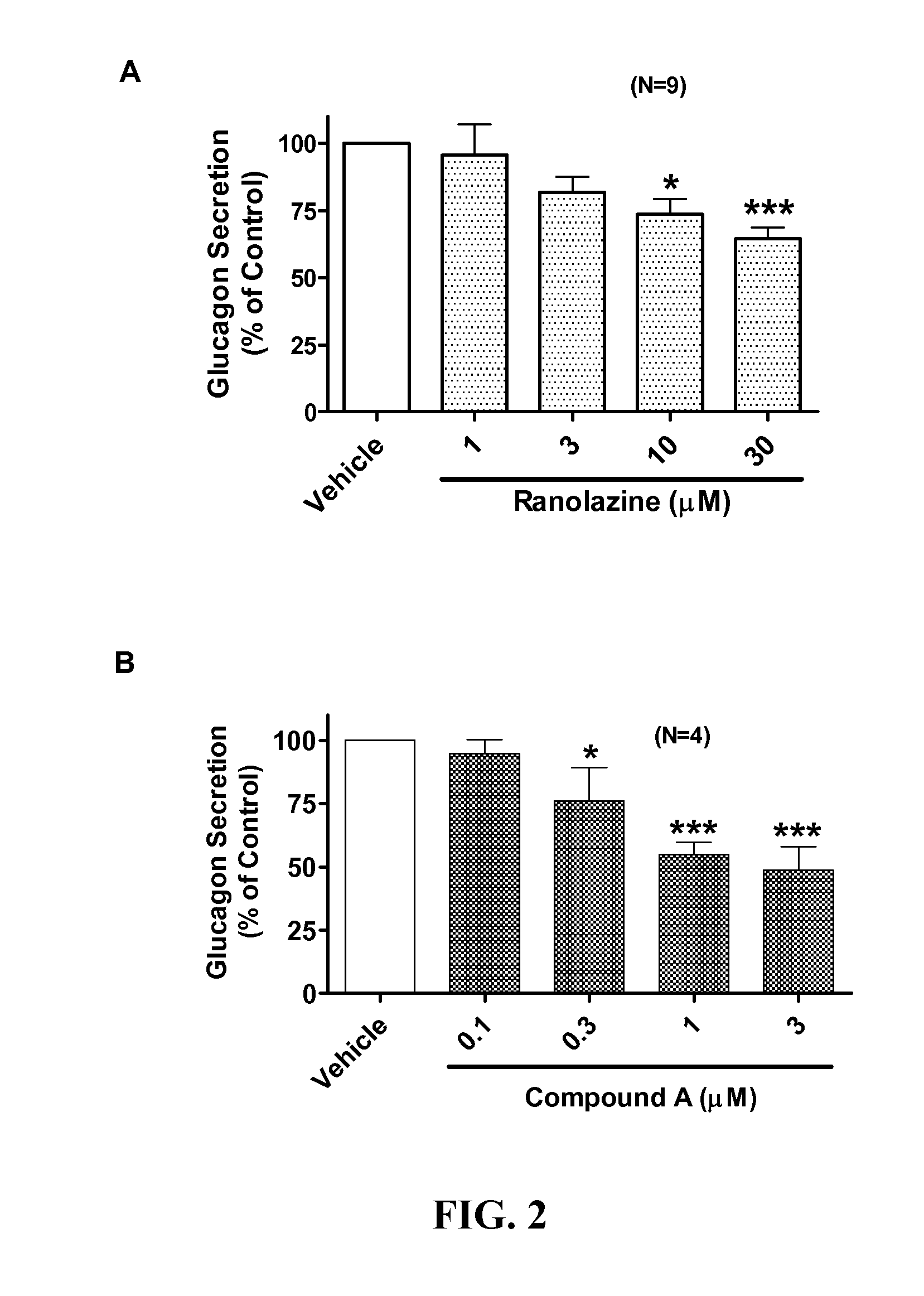
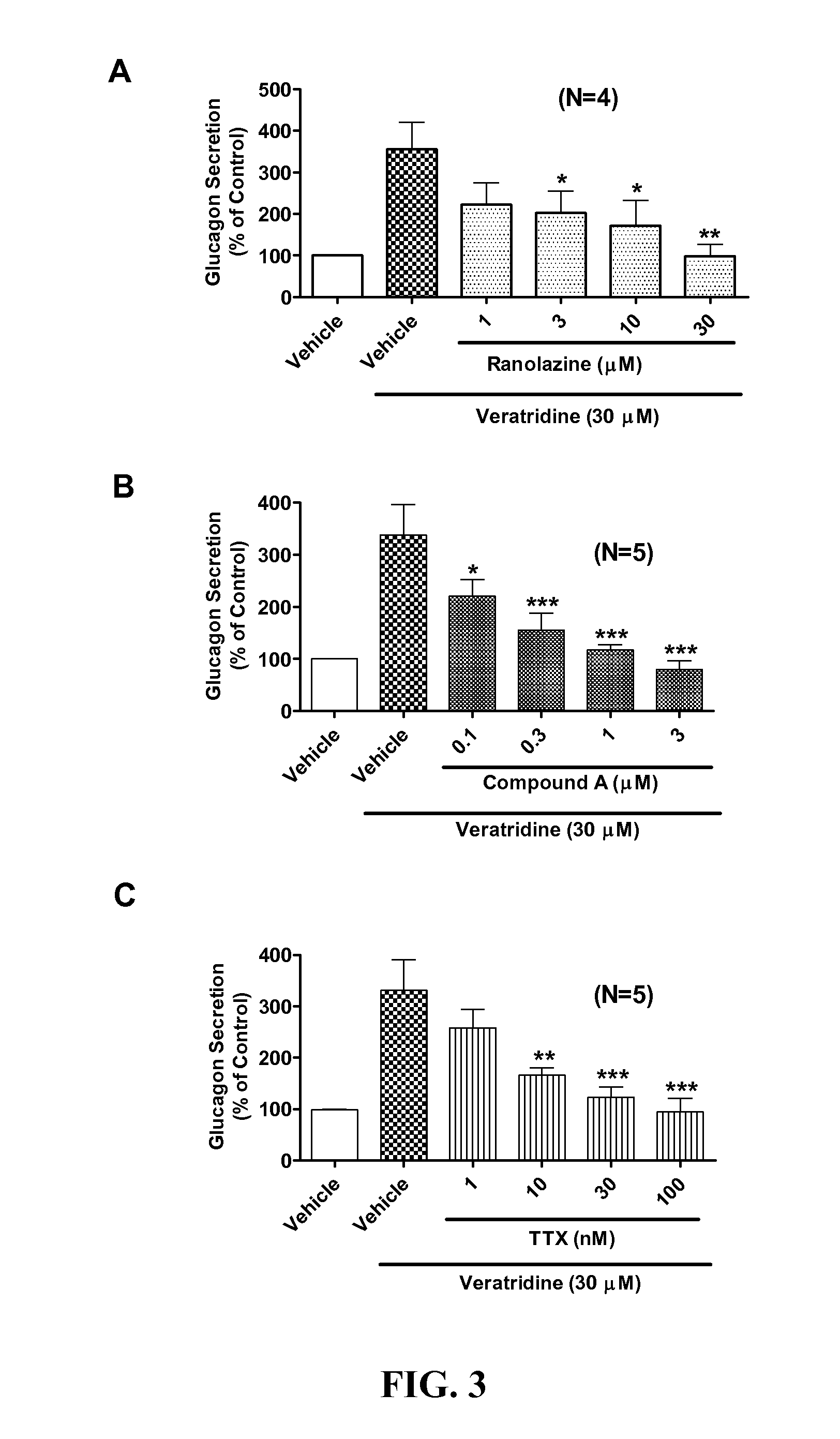
Sodium channel blockers reduce glucagon secretion
InactiveUS20140221286A1Increase contentHigh currentBiocidePeptide/protein ingredientsDiseaseAcute hyperglycaemia
Owner:GILEAD SCI INC
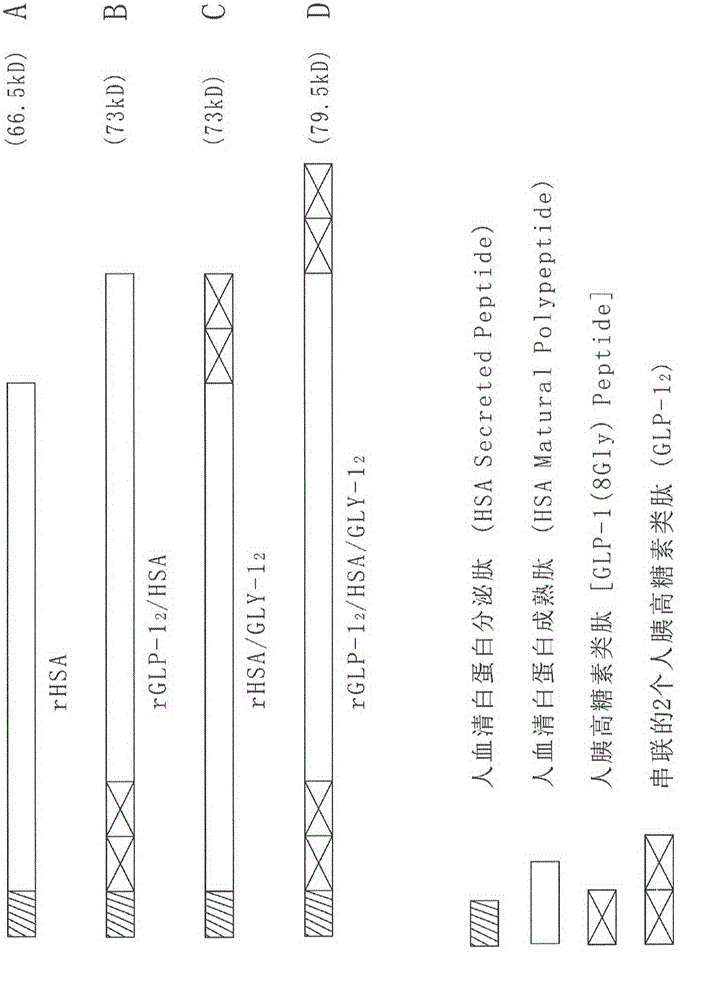
Recombinant human serum albumin/pancreatic glucagon peptide fusion protein having blood sugar content continuous control function
The invention relates to a novel molecular drug structure of recombinant human serum albumin / pancreatic glucagon peptide fusion protein capable of obviously reducing blood sugar content in blood and having continuous effect: rGLP-12 / HSA / GLP-12. Especially, the invention uses a genetic engineering technology for constructing the engineered yeast strain to express the produced recombinant fusion protein. The recombinant fusion protein has the advantages that 1) blood sugar content in the blood is reduced, body weight is reduced, and the recombinant fusion protein is used for treating diabetes and cardiovascular disease; 2) life of pancreatic glucagon peptide is greatly prolonged in vitro and vivo, so that the recombinant fusion protein has maximum stability in the blood and can be continuously effected on the blood sugar control; and 3) saccharomycetes can be used for realizing low-cost large-scale production and preparation process.
Owner:BEIJING MEIFUYUAN BIO PHARM TECH +2
Glucagon Receptor Modulators
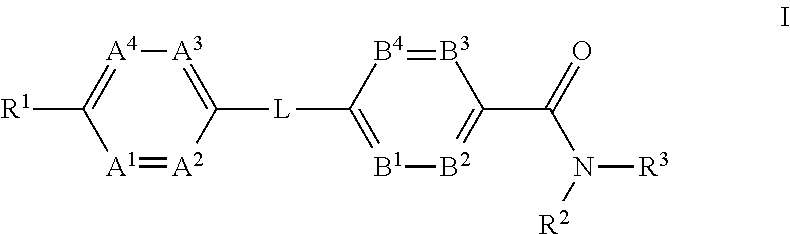
InactiveUS20120165343A1Lower blood sugar levelsBiocideSenses disorderStereochemistryGlucagon receptor
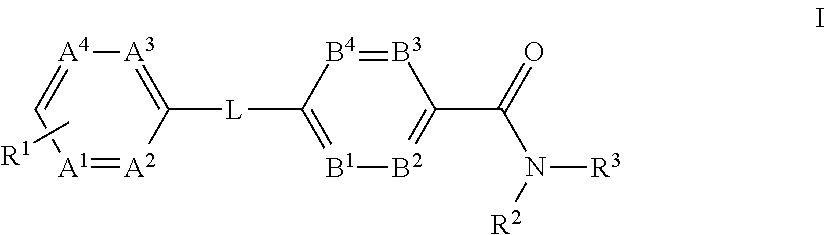
The present invention provides a compound of Formula (I)or a pharmaceutically acceptable salt thereof wherein R1, R2, R3, A1, A2, A3, A4, L, B1, B2, B3 and B4 are as defined herein. The compounds of Formula I have been found to act as glucagon antagonists or inverse agonists. Consequently, the compounds of Formula I and the pharmaceutical compositions thereof are useful for the treatment of diseases, disorders, or conditions mediated by glucagon.
Owner:PFIZER INC
Long-acting co-agonists of the glucagon and GLP-1 receptors
Owner:MERCK SHARP & DOHME LLC
Glucagon analogue, and preparation method and application thereof
The invention relates to the technical field of biology, specifically to a glucagon analogue, and a preparation method and an application thereof. The glucagon analogue provided by the invention comprises a glucagon-like polypeptide fragment, wherein a long-acting carrier is cross-linked on the glucagon-like polypeptide fragment. According to the glucagon analogue provided by the invention, only 2-3 amino acids of a polypeptide chain are mutated on the basis of a natural Glucagon sequence, and the risk of immunogenicity is extremely low, so a C-terminal sequence of a listed drug Exenatide (with a trade name of Bydureon) is additionally introduced, and higher security is achieved. Furthermore, the glucagon analogue provided by the invention has extremely high GLP-1R and GCGR agonistic activity, and surprisingly, the glucagon analogue has significant change in in-vitro activity before and after fatty acid crosslinking.
Owner:HANGZHOU HEZE PHARMA TECH +1
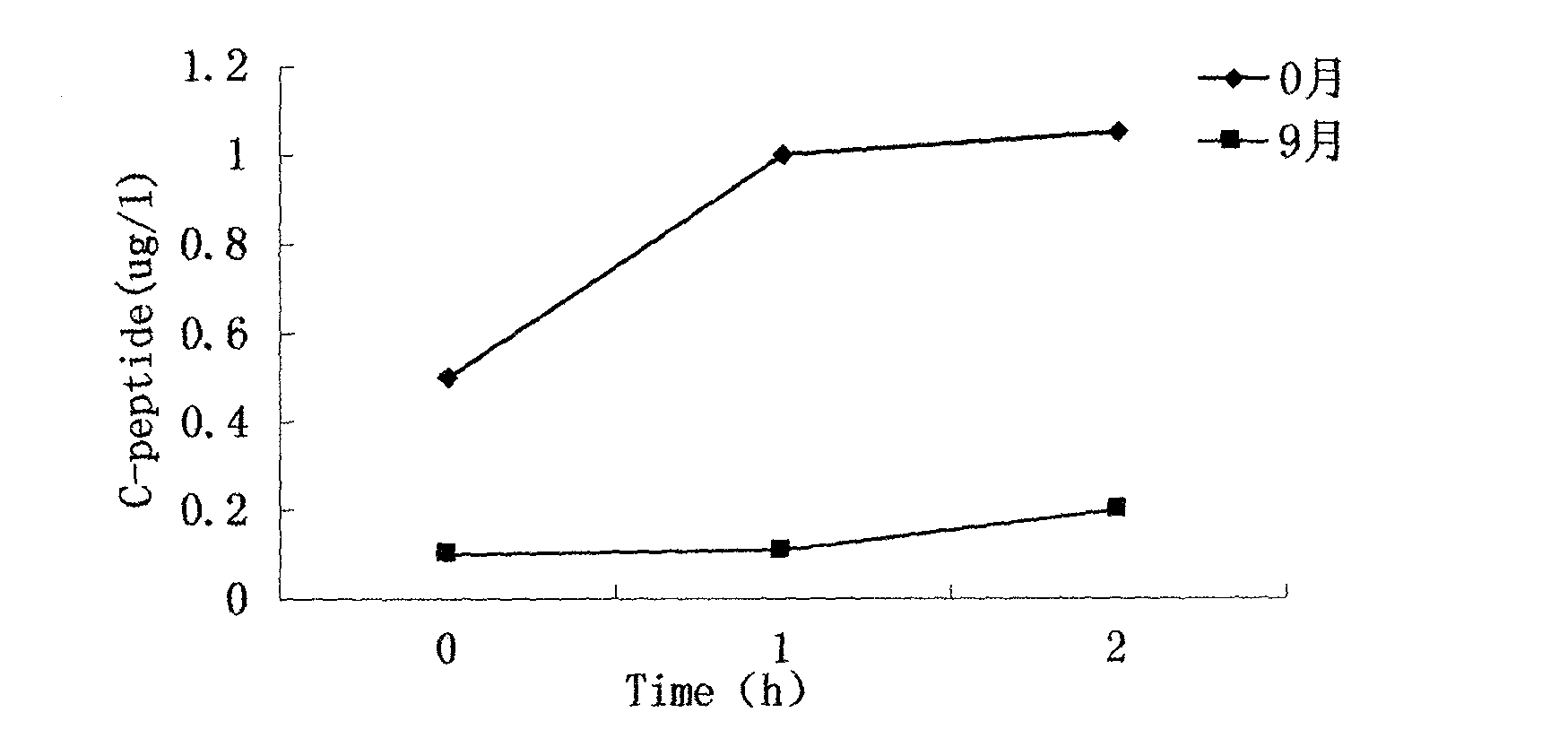
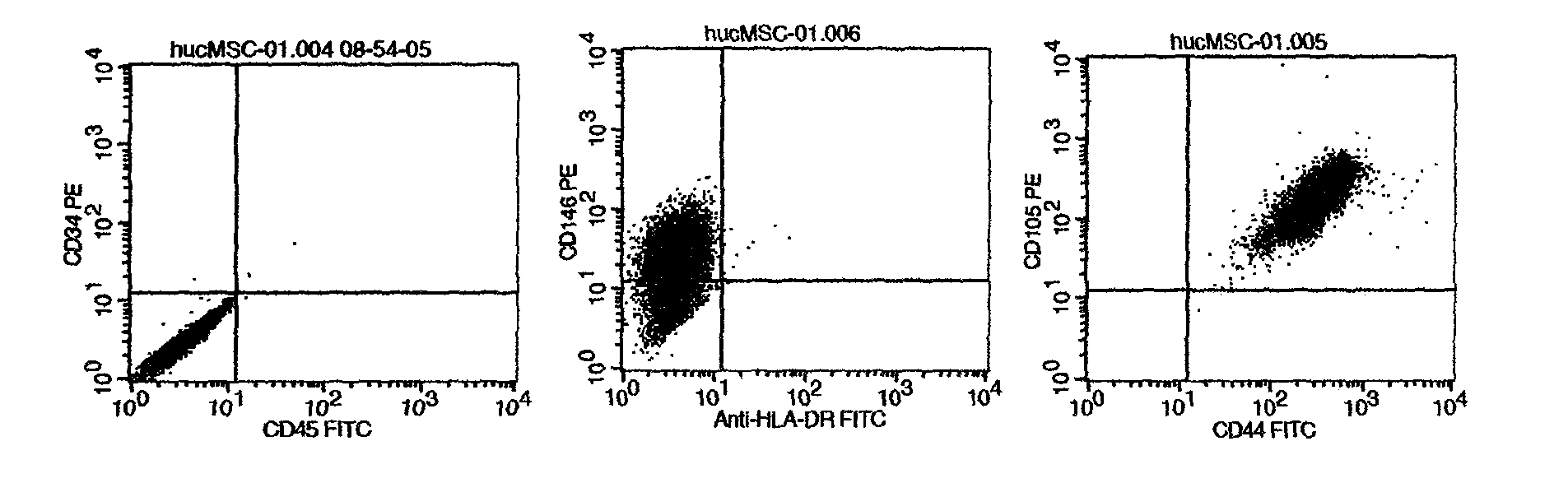
New method provided by combination of human umbilical cord mesenchymal stem cells and incretin for improvement of functions of diabetes islet beta cells
InactiveCN103566358APromote hyperplasiaPromote regenerationPeptide/protein ingredientsMetabolism disorderDipeptidyl peptidaseBeta-cell Function
A new method for improvement of functions of diabetes islet beta cells is provided by combination of human umbilical cord mesenchymal stem cells and incretin, and the new method is as follows: on the basis of application of the human umbilical cord mesenchymal stem cells for treatment of type-1 and type-2 diabetes, the incretin (GLP-1 (glucagon-like peptide-1) receptor agonist and DPP-4 (dipeptidyl peptidase-4) inhibitor) is combined. The new method can promote islet beta cell proliferation and regeneration, improve the functions of the islet beta cells, reduce blood sugar and glycated hemoglobin, reduce islet alpha hyperplasia and inhibit glucagon secretion, and thus playing a synergy effect.
Owner:HOSPITAL ATTACHED TO QINGDAO UNIV
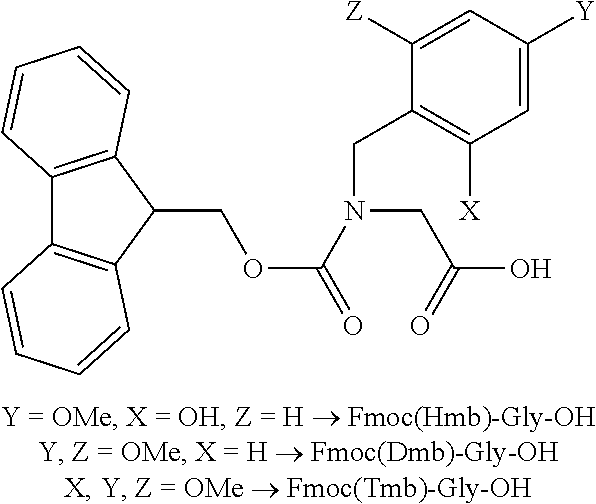
Process for the preparation of high purity glucagon
ActiveUS20200399339A1Speed up the processHigh yieldPeptide-nucleic acidsPeptide/protein ingredientsPancreatic glucagonOrganic chemistry
The present invention provides an improved process for the preparation of high purity glucagon comprising the use of Xmb-protected amino acids, wherein may Xmb include, e.g., 2,4,6-trimethoxybenzyl, 2,4-dimethoxybenzyl, or 2-hydroxy-4-methoxybenzyl. The process also comprises the use of building blocks such as pseudoprolines to avoid aggregation and obtain the product in high yield and purity.
Owner:FRESENIUS KABI IPSUM SRL
Novel stable preparation of recombinant human pancreatic glucagon-like peptide-1 analogue fusion protein
The invention describes a novel stable preparation of a recombinant human pancreatic glucagon-like peptide-1 analogue fusion protein. The medicinal preparation can be used for treating diabetes and related diseases.
Owner:JIANGSU T MAB BIOPHARMA
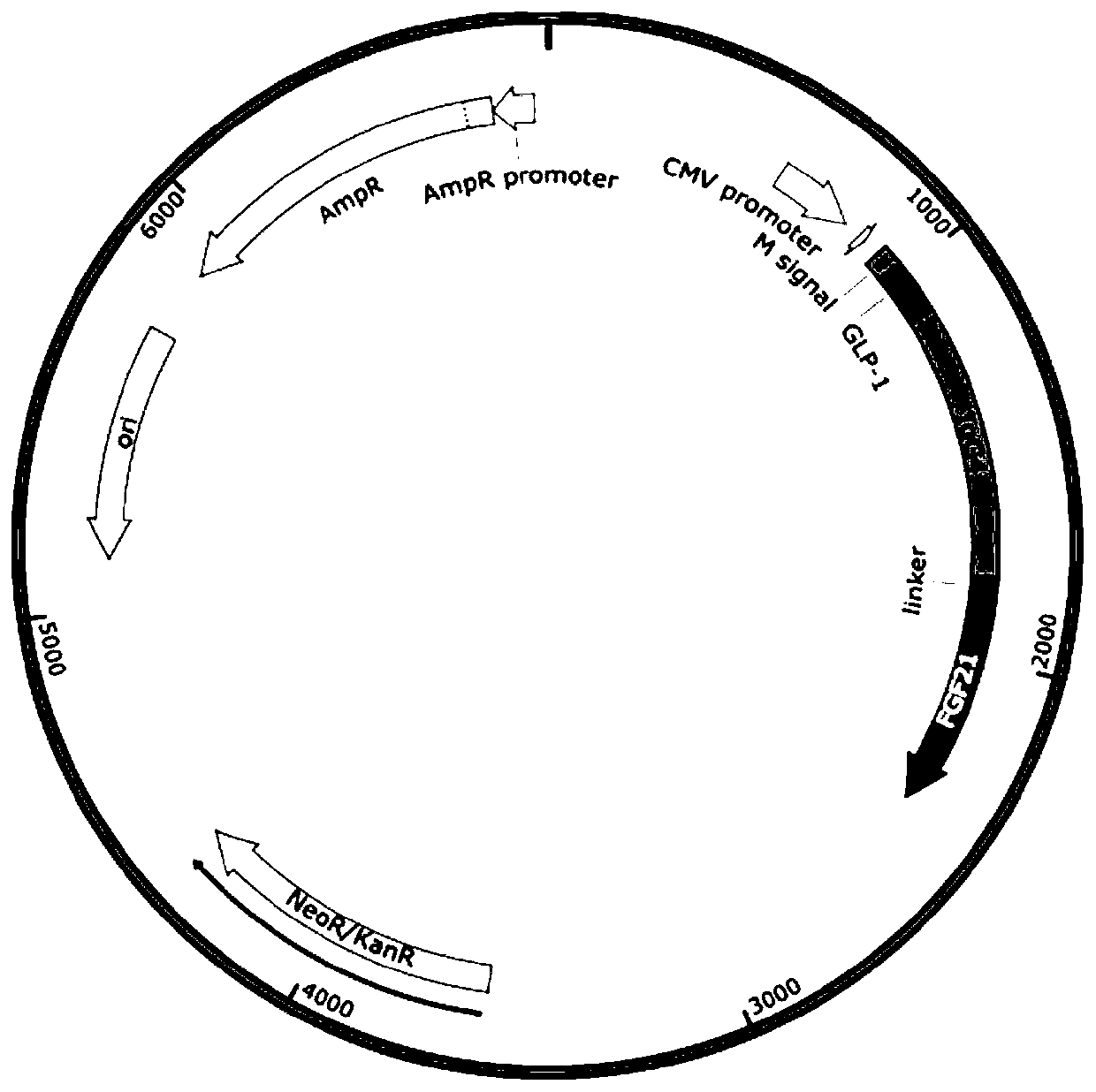
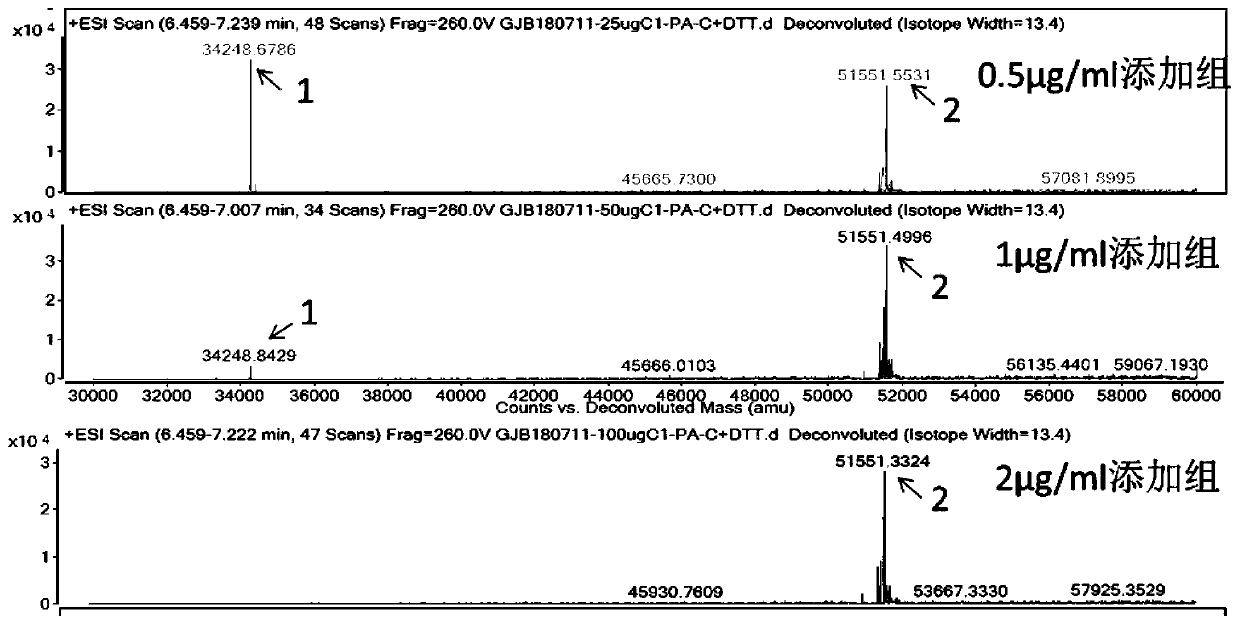
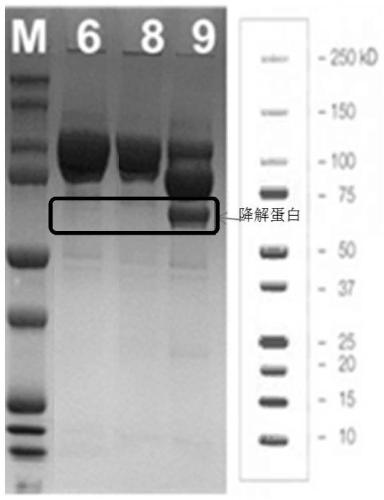
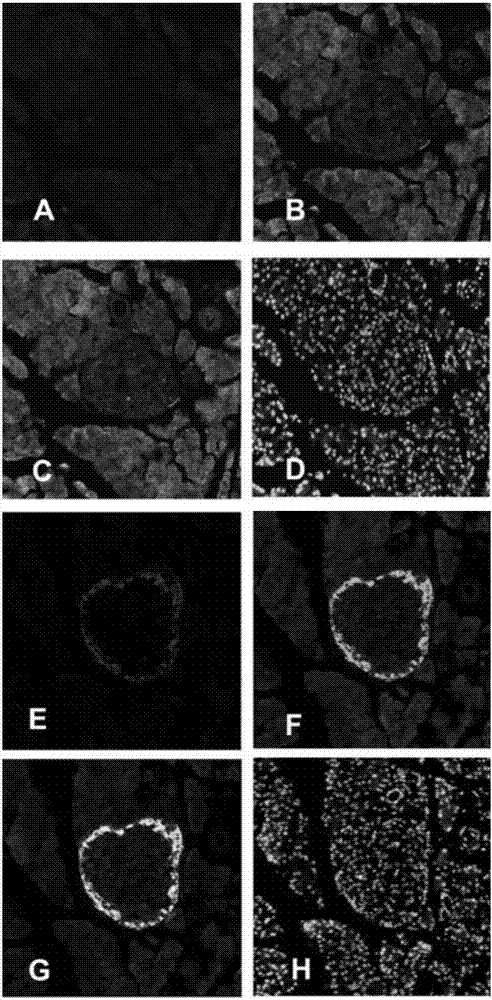
FGF21 Fusion Proteins and Method of Inhibiting Degradation Thereof
ActiveCN111087475AAvoid degradationThe degradation effect is stableAntibody mimetics/scaffoldsGenetically modified cellsVirus inhibitorsPancreatic glucagon
The invention discloses an FGF21 fusion protein. The fusion protein comprises (1) a glucagon-like polypeptide 1 (GLP-1) variant of the amino acid sequence shown in SEQ ID NO: 4, a connecting peptide of the amino acid sequence shown in SEQ ID NO: 6, a Fc portion of human IgG4 variant antibody having amino acid sequence shown in SEQ ID NO: 8 and a fibroblast growth factor 21 (FGF21) variant of the amino acid sequence set forth in SEQ ID NO: 10; or (2): a fusion protein which is derived from (1) by substituting, deleting or adding one or more amino acids in the amino acid sequence in (1) and hasthe activity of the FGF21 fusion protein. The invention further discloses a method for inhibiting degradation of the FGF21 fusion protein. The method comprises the step of adding the C1 protease inhibitor into a culture medium. The method provided by the invention can effectively inhibit the degradation of the FGF21 fusion protein.
Owner:DONGGUAN HEC BIOPHARMACEUTICAL R&D CO LTD
Application of pancreatic GnRH receptor function regulator to preparation of drugs used for treating type 2 diabetes
InactiveCN107412769ASecretory peak delayInhibition of secretionMetabolism disorderPharmaceutical active ingredientsHigh concentrationConcentrations glucose
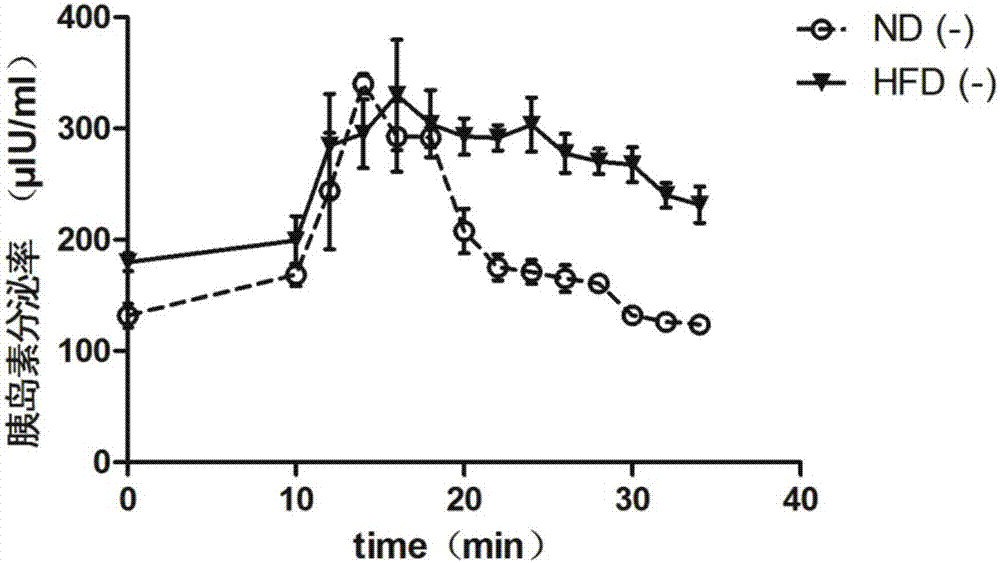
The invention relates to application of a pancreatic GnRH receptor function regulator to preparation of drugs used for treating type 2 diabetes. According to the invention, pancreases with normal glucose concentration and pancreases with high-concentration glucose for simulating diabetes are subjected to perfusion of GnRH and a pancreatic GnRH receptor, and results show that pancreatic GnRH with a certain concentration has important regulating effect on the secretion time phase of main hormones of pancreatic islets, i.e., insulin and pancreatic glucagon and that a perfusion solution with certain-concentration GnRH does not inhibit the secretion of pancreatic glucagon, stimulates the secretion of pancreatic glucagon instead and leads to delay of the secretion peak of insulin. Thus, a theoretical and experimental foundation is provided for preparation of drugs for regulating the secretion time phase of insular hormones in virtue of pancreatic GnRH and regulating substances for the receptor approach of pancreatic GnRH and further for preparation of drugs used for restoring inhibition of high glucose on pancreatic glucagon in virtue of pancreatic GnRH and the receptor approach thereof.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Stabilized glucagon nanoemulsions
The present invention provides an oil-in-water nanoemulsion containing glucagon, an oily phase, and an aqueous phase, wherein the glucagon is physically and chemically stable and the nanoemulsion is suitable for administration by manual injection or by a pump to treat hypoglycemia.
Owner:陈献
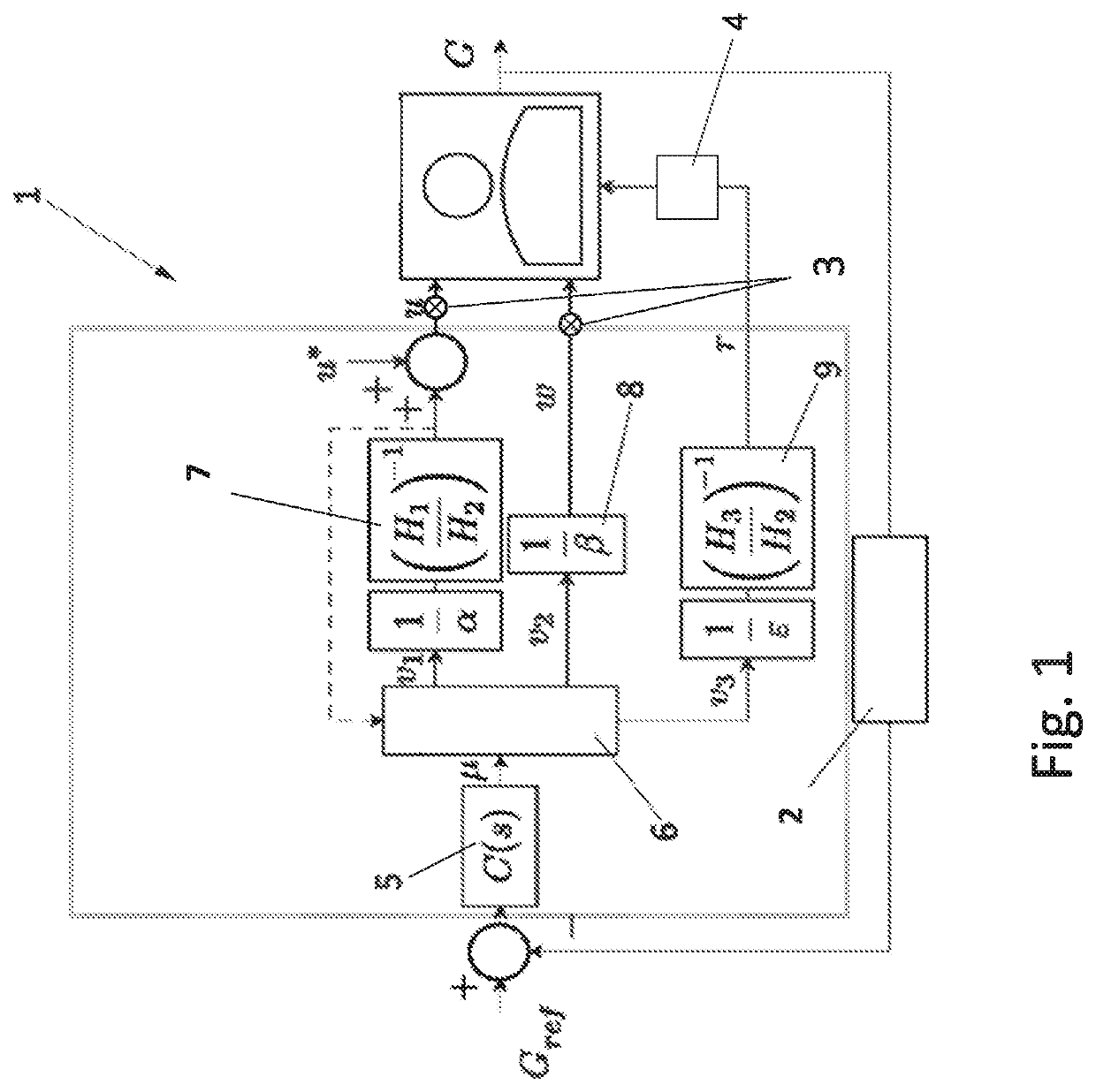
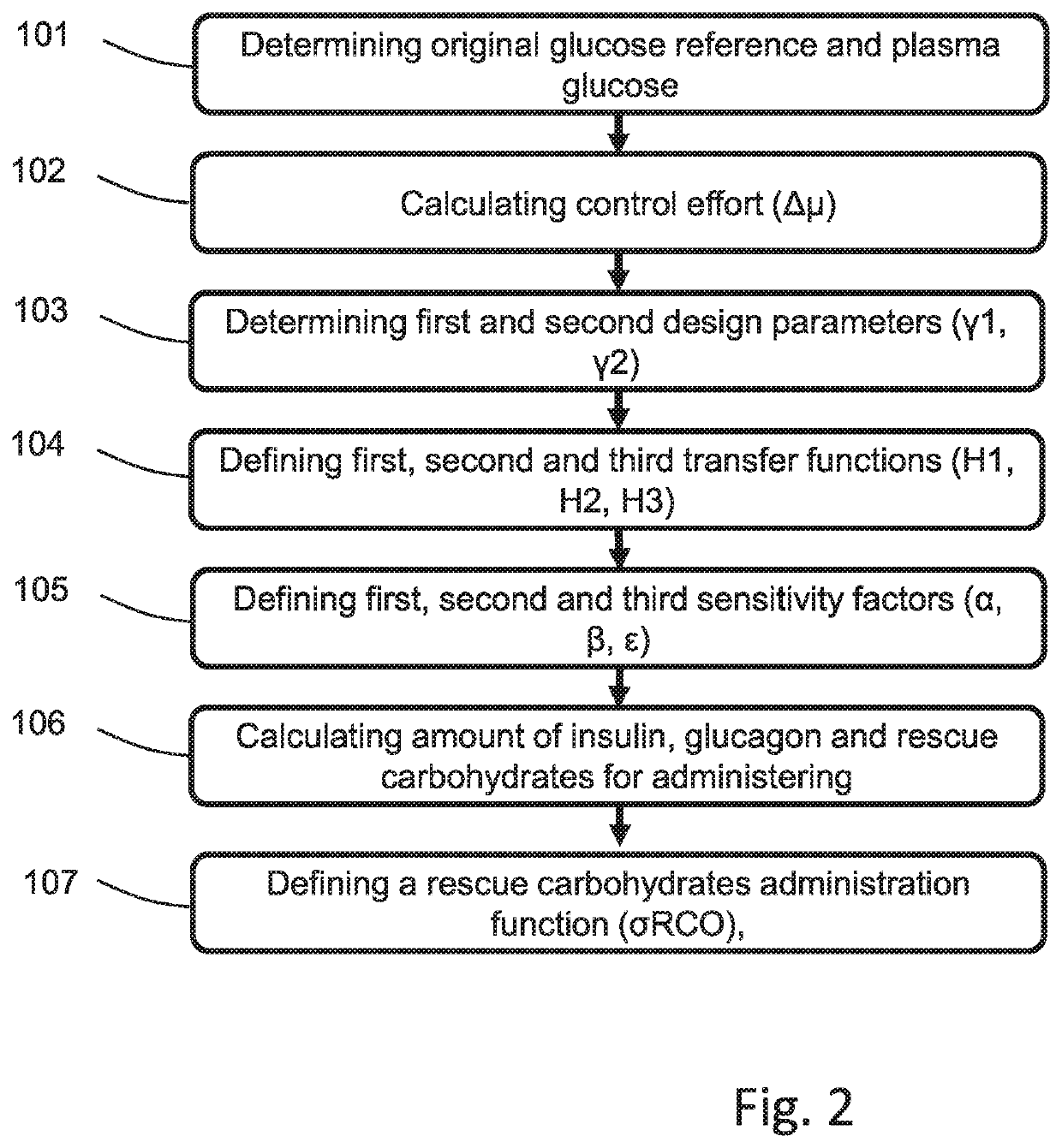
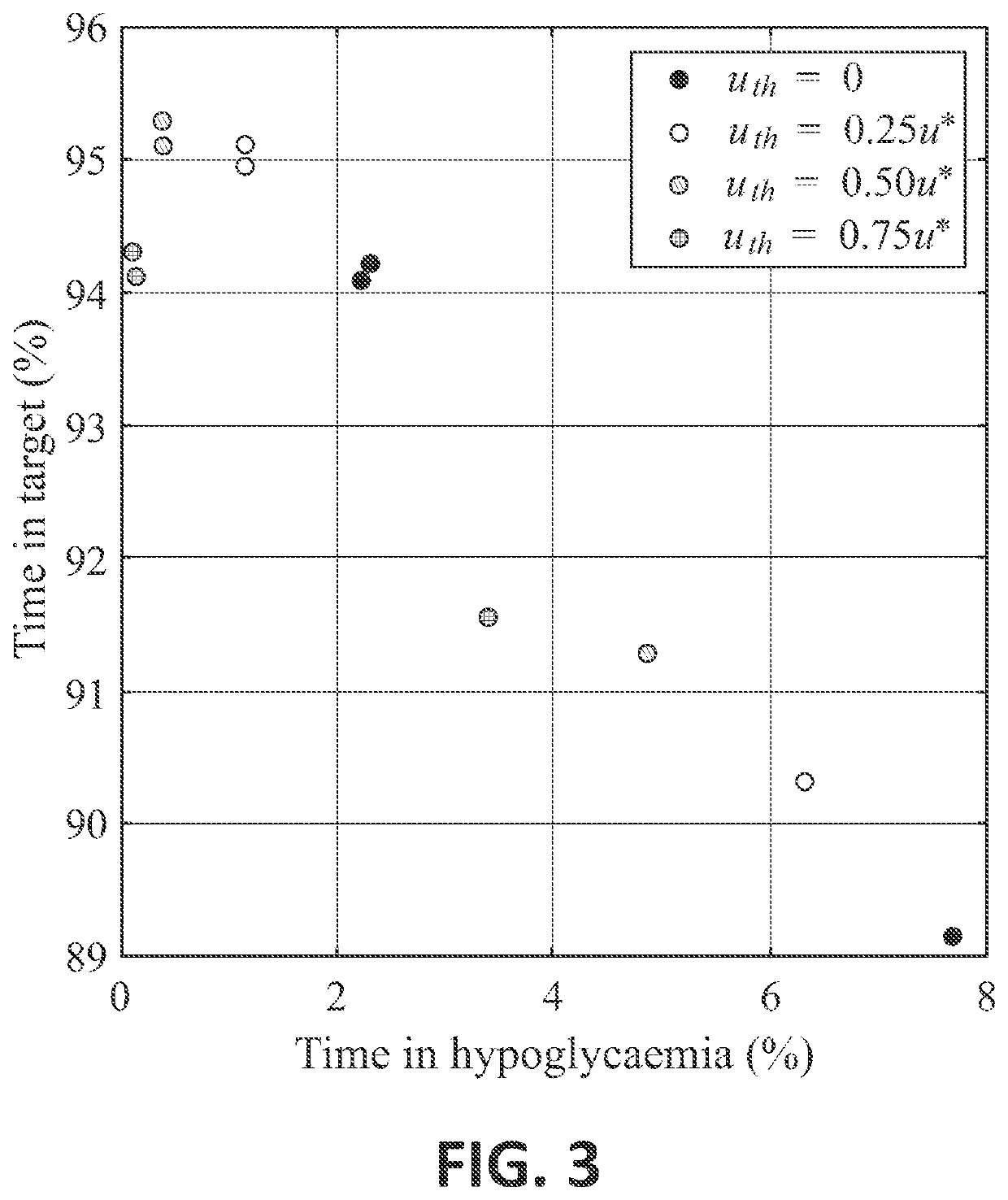
Control Method and Artificial Pancreas for Administration of Insulin, Glucagon and Rescue Carbohydrates
PendingUS20220044783A1Effective controlMedical simulationMedical data miningPhysiologyPancreatic hormone
Control method for glucose control by administrating insulin, glucagon and rescue carbohydrate includes the steps of determining a glucose reference and plasma glucose; calculating a control effort from the glucose reference and the plasma glucose; defining a first design parameter and a second design parameter being the first design parameter the relative weight between control actions and counterregulatory actions, and the second design parameter the relative weight between the counterregulatory actions; defining transfer functions representing the glycemic effect of administrating insulin, glucagon and rescue carbohydrate, normalized to unit gain; defining sensitivity factors representing the sensitiveness of a patient to insulin, glucagon and rescue carbohydrates; and calculating the rate of insulin, glucagon and rescue carbohydrate for administering by distributing the calculated control effort.
Owner:UNIV POLITECNICA DE VALENCIA
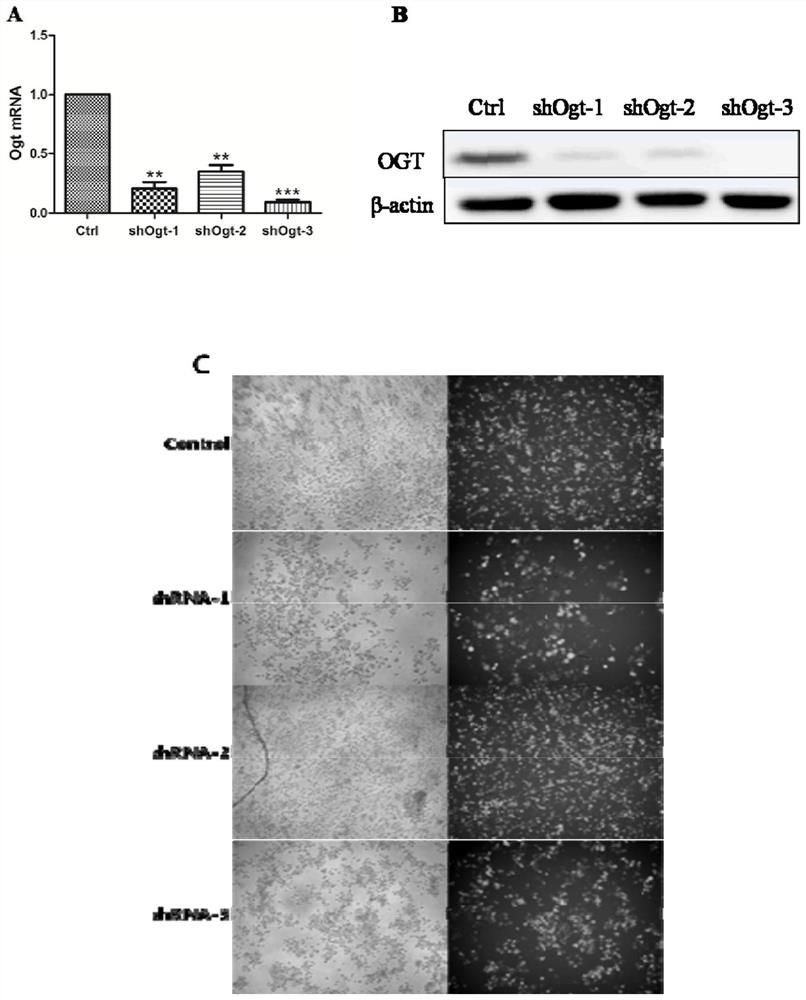
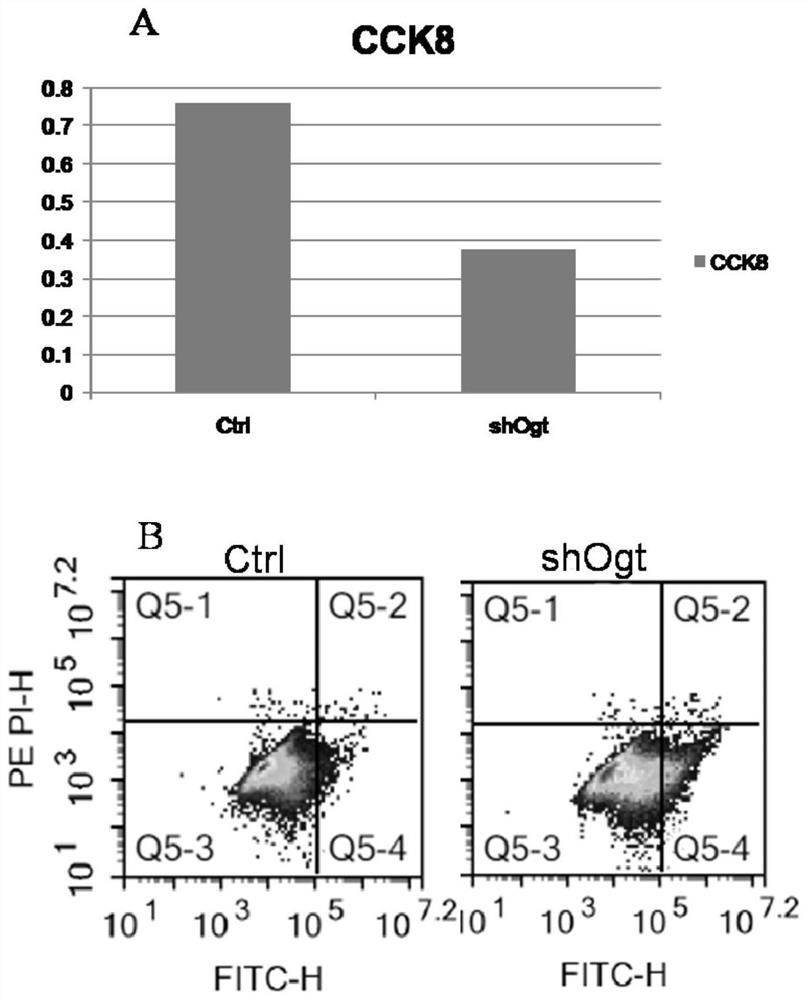
Application of OGT as target in preparation of medicine for treating abnormal secretion of glucagon in diabetes
InactiveCN112891540AReduce secretionPromote witheringMetabolism disorderMicrobiological testing/measurementAntiendomysial antibodiesIslet cells
The invention discloses application of OGT as a target in preparation of a medicine for treating abnormal secretion of glucagon in diabetes. The medicine is a medicine for inhibiting expression of O-GlcNAc glycosyltransferase OGT, wherein the functional component can be at least one of shRNA, siRNA, dsRNA, miRNA, cDNA, antisense RNA / DNA, a low molecular compound, peptide and an antibody. Studies find that the secretion amount of glucagon can be reduced by knocking out or inhibiting O-GlcNAc glycosyltransferase OGT, meanwhile, withering of islet alpha cells can be promoted, proliferation of the islet alpha cells is inhibited, and O-GlcNAc glycosyltransferase OGT can be used as a target for treating diabetes.
Owner:BINZHOU MEDICAL COLLEGE
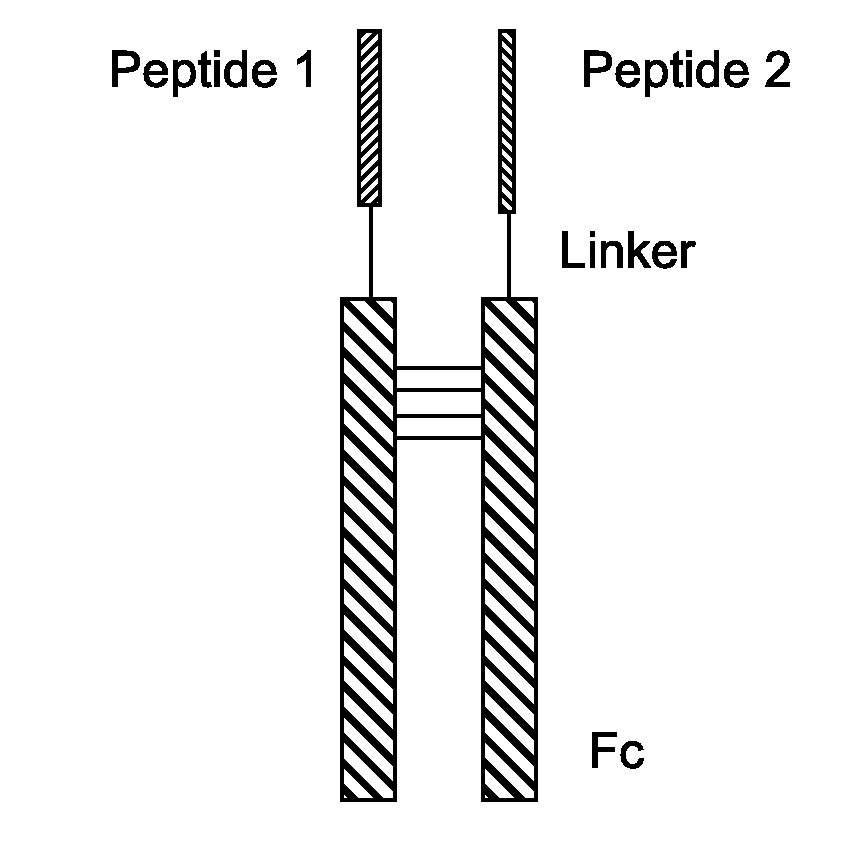
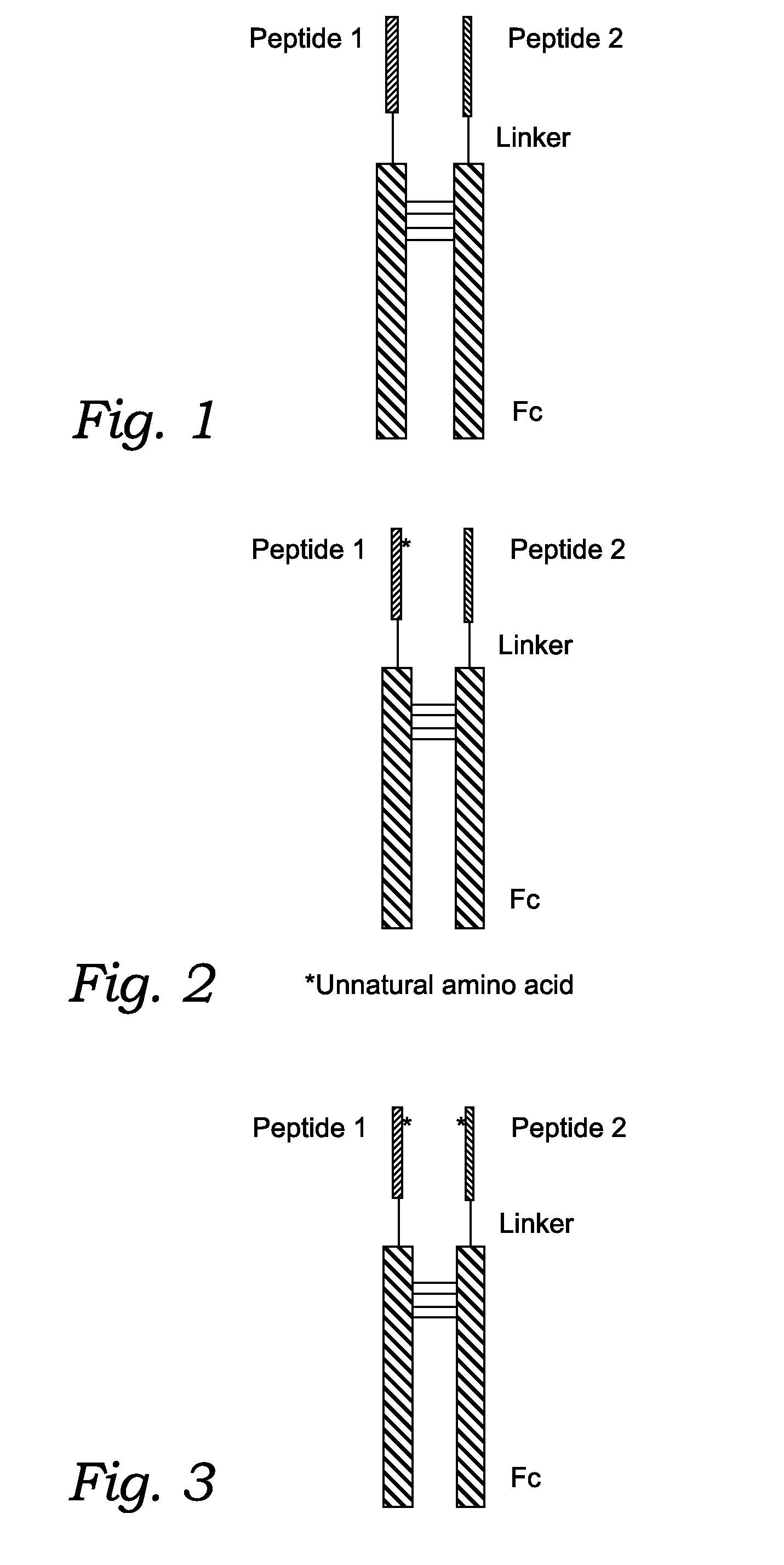
Fusion Proteins With Dual Receptor Agonist Activities
InactiveUS20160137712A1Short half-lifeSmall molecular weightPeptide/protein ingredientsAntibody mimetics/scaffoldsDimerPancreatic glucagon
The present disclosure relates to heterodimeric fusion proteins comprising two polypeptides, the first polypeptide comprising a first peptide (P1), a linker (L1), and a Fc region (F1), the second polypeptide comprising a second peptide (P2), a linker (L2), and an Fc region (F2), wherein P1 and P2 are each independently selected from GLP-1, GLP-1 analogues, glucagon, glucacon analogues, GIP, GIP analogues, oxyntomodulin, oxyntomodulin analogues, exendin and exendin analogues; wherein F is selected from an IgG Fc, an IgA Fc, an IgE Fc, an IgGM Fc, and their analogues; wherein the C-terminals of the peptides are linked, though the Linker L, to the N-terminals of the Fc region F. In one embodiment, the fusion proteins disclosed herein have agonist activity against at least two of the GLP-1 receptor, the GIP receptor, and the glucagon receptor.
Owner:ASKGENE PHARM INC +1
Method for preparing human pancreatic glucagons like polypeptide - 1, and fusion proten of human serum albumin, and products
InactiveCN1916173AAvoid degradationAvoiding Immunogenicity IssuesFungiFermentationHalf-lifeGlucagon-like peptide-1
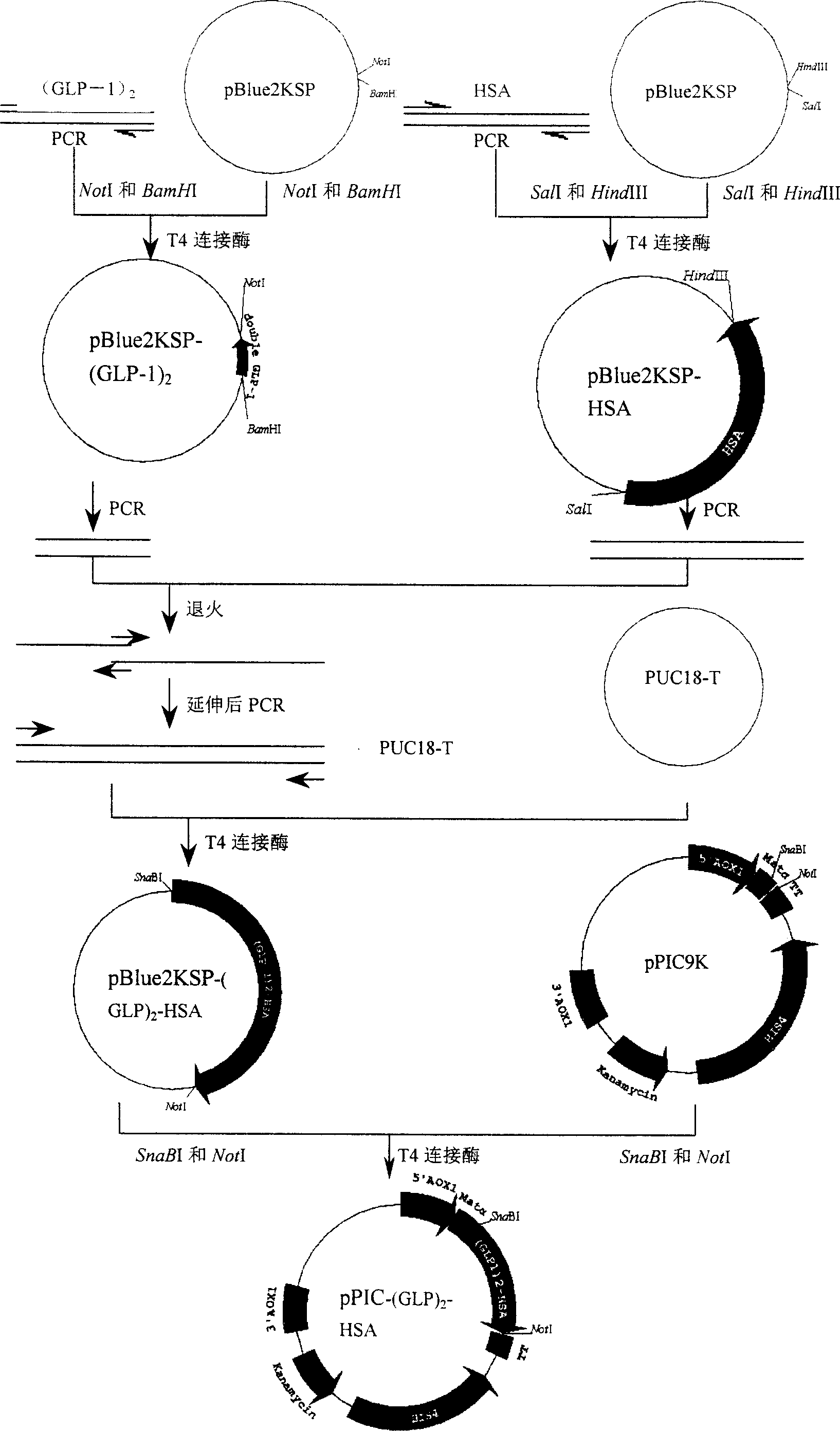
This invention discloses a method for preparing fusion protein and its related products containing human glucagon-like peptide 1 (GLP-1) and human serum albumin (HSA). The fusion protein contains a first peptide having at least 85% homology to human GLP-1 and a second peptide having at least 85% homology to HSA, wherein the two peptides are directly linked without any linking peptide, and several amino acid residues can be replaced, deleted or inserted if not changing the property of the fusion protein. The fusion protein is obtained by linking two tandem GLP-1 cDNA ((GLP-1)2) and HAS cDNA through overlapping PCR and expressing the fusion gene ((GLP-1)2-HAS) in a host cell. The fusion protein retains the physicological property of human glucagon-like peptide 1, and has improved half-life in vivo and potential application in pharmaceutical field.
Owner:JIANGNAN UNIV
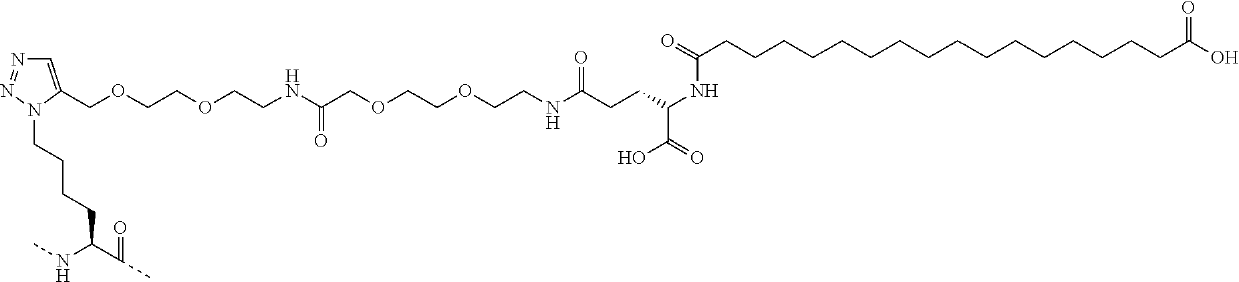
Glucagon derivative and a composition comprising a long acting conjugate of the same
ActiveUS20180186853A1Good physical propertiesImprove compliancePeptide/protein ingredientsMetabolism disorderStereochemistryLong acting
Owner:HANMI PHARMA
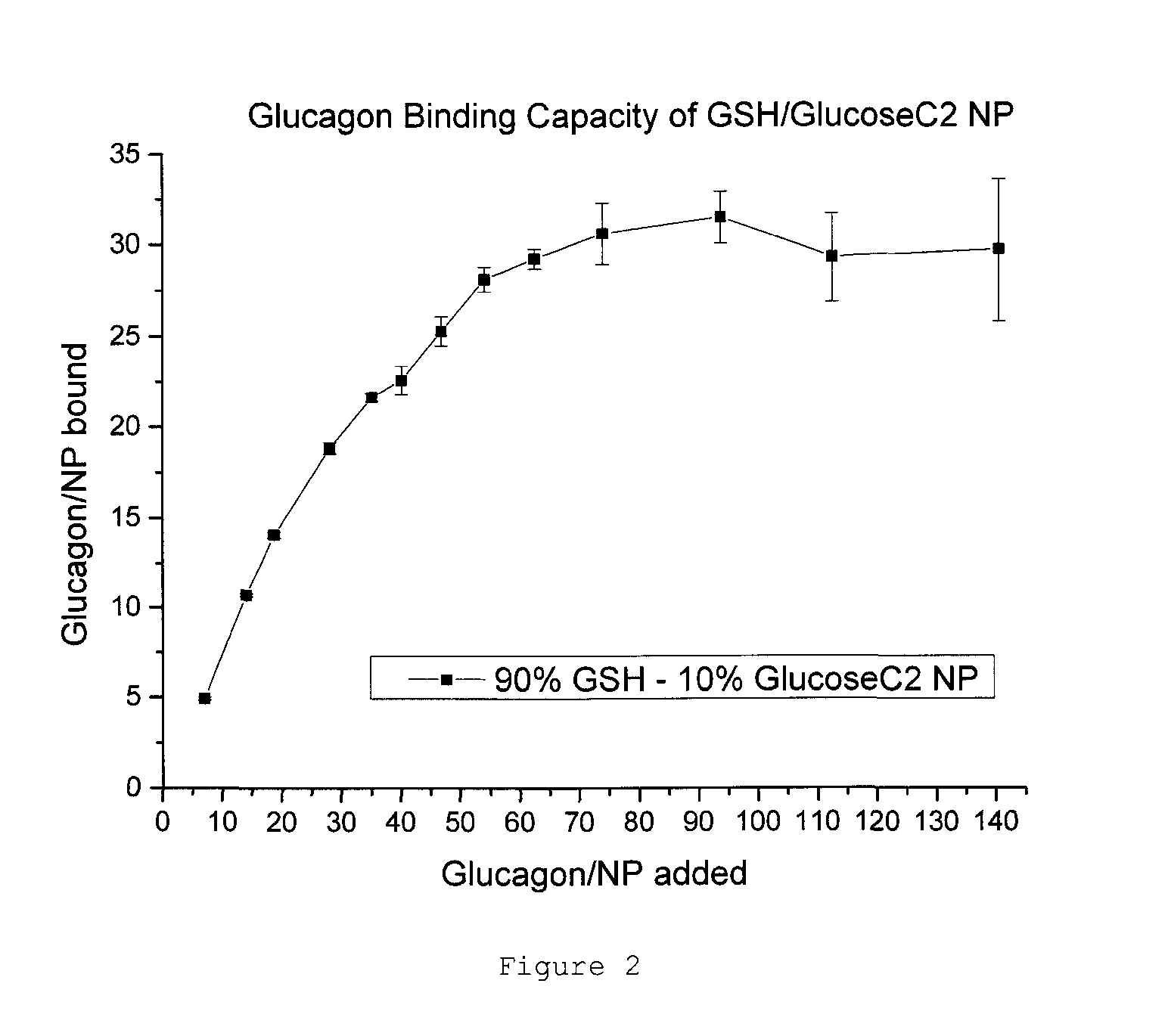
Nanoparticle glucagon compositions
InactiveUS20150216940A1Easy to manageQuick managementPowder deliveryPeptide/protein ingredientsNanoparticleMedicine
The present invention relates to glucagon peptide-carrying nanoparticles, particularly for use in medicine, and includes methods for treatment of hypoglycaemia, such as a diabetic hypoglycaemic adverse event. Nanoparticle composition comprise a nanoparticle comprising a core comprising a metal and / or a semiconductor; and a corona comprising a plurality of ligands covalently linked to the core, wherein said plurality of ligands comprise at least one glutathione; and at least one glucagon peptide that is non-covalently bound to the corona.
Owner:MIDATECH LTD
Glucagon derivative and a composition comprising a long acting conjugate of the same
ActiveUS10696725B2Prolong half-life in vivoGood physical propertiesPeptide/protein ingredientsMetabolism disorderPancreatic glucagonMedicinal chemistry
Owner:HANMI PHARMA
Treatment of post-bariatric hypoglycemia using mini-dose stable glucagon
PendingUS20210030847A1Reduced likelihoodReduce severityPeptide/protein ingredientsMetabolism disorderExtracorporeal bypassIntestine bypass
Post-bariatric hypoglycemia (PBH) is an increasingly-recognized complication of gastric bypass surgery. Current therapeutic options have suboptimal efficacy. Small doses of stable liquid glucagon can be used to treat or prevent post-bariatric hypoglycemia.
Owner:JOSLIN D ABETES CENTER INC +1
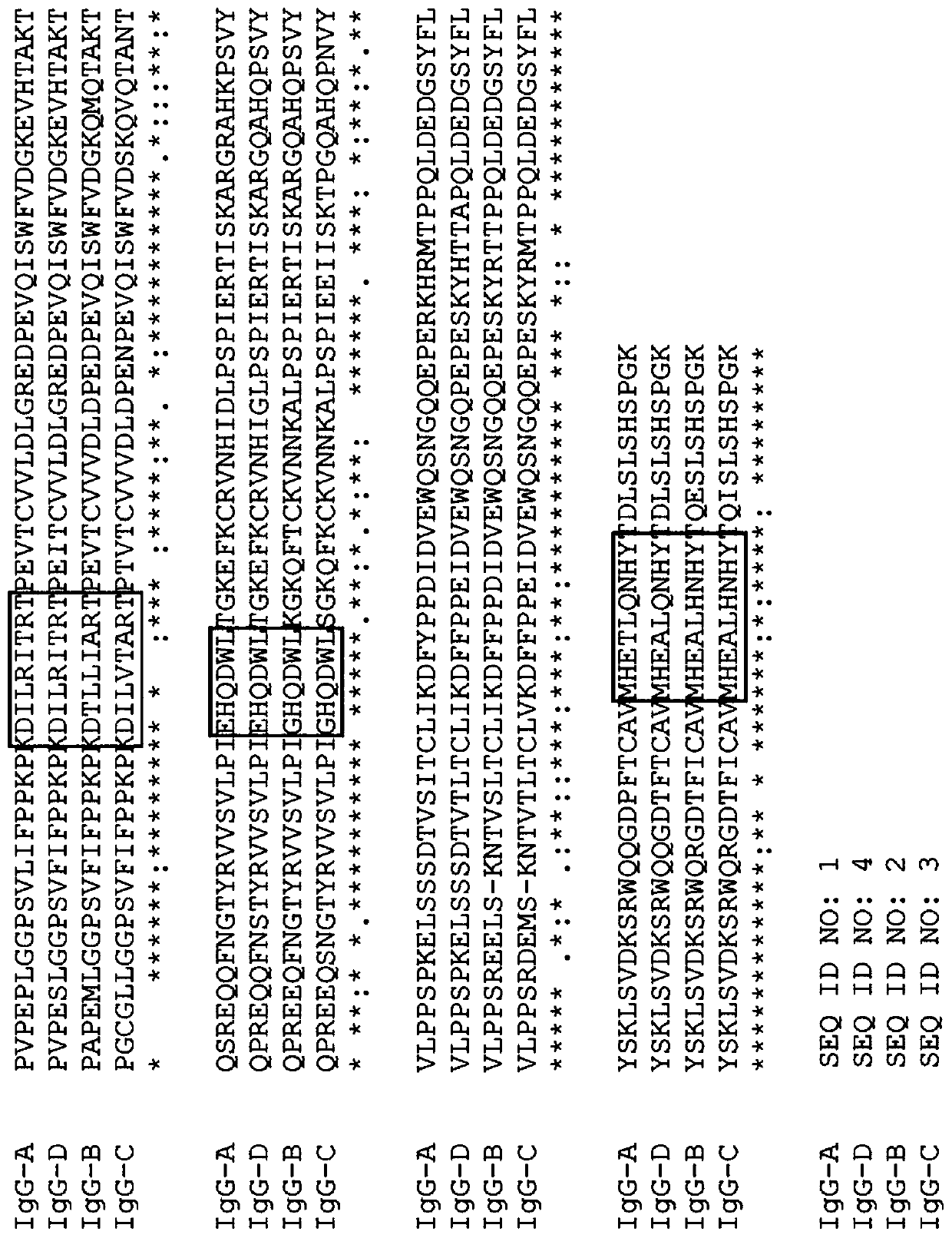
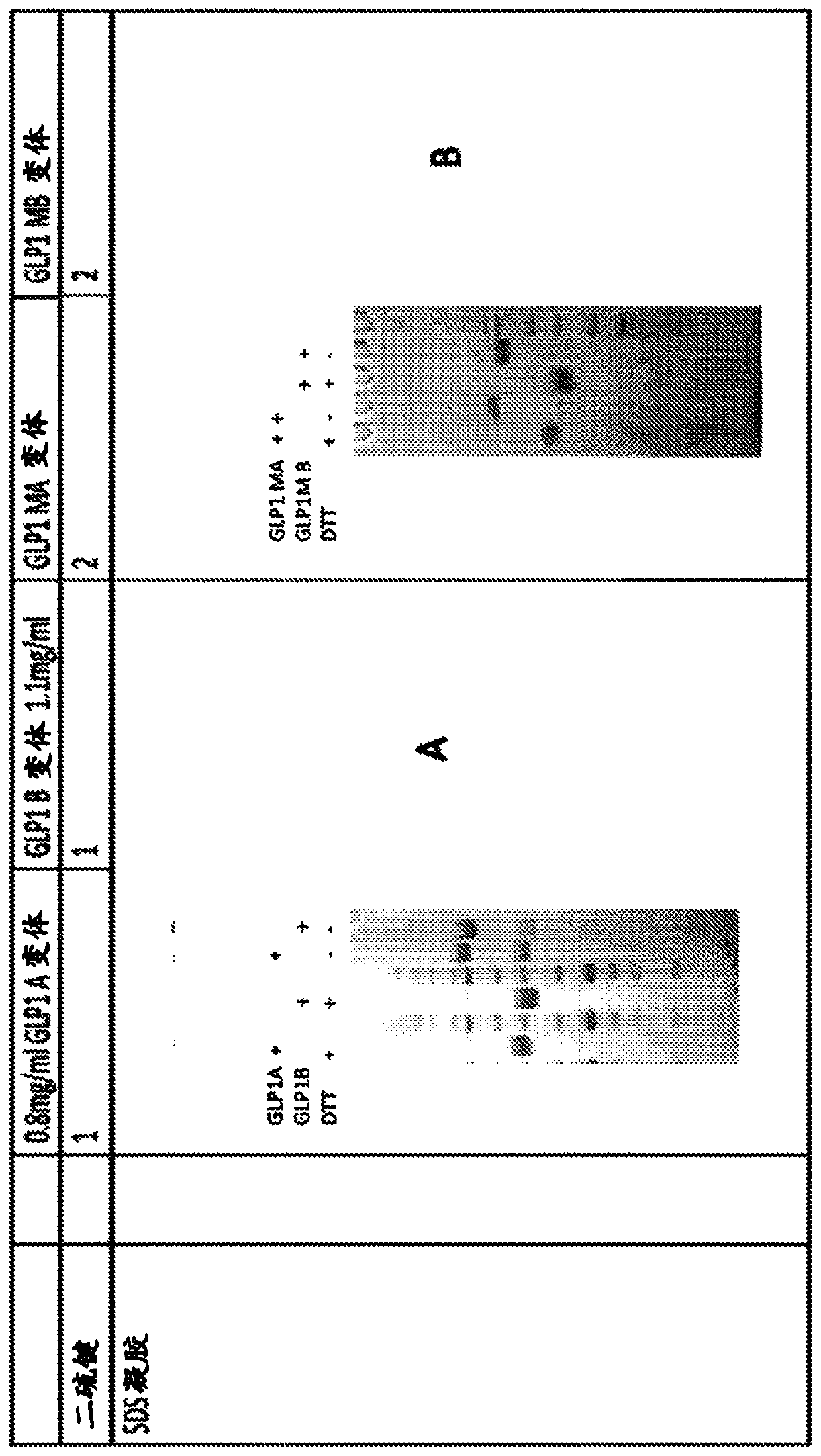
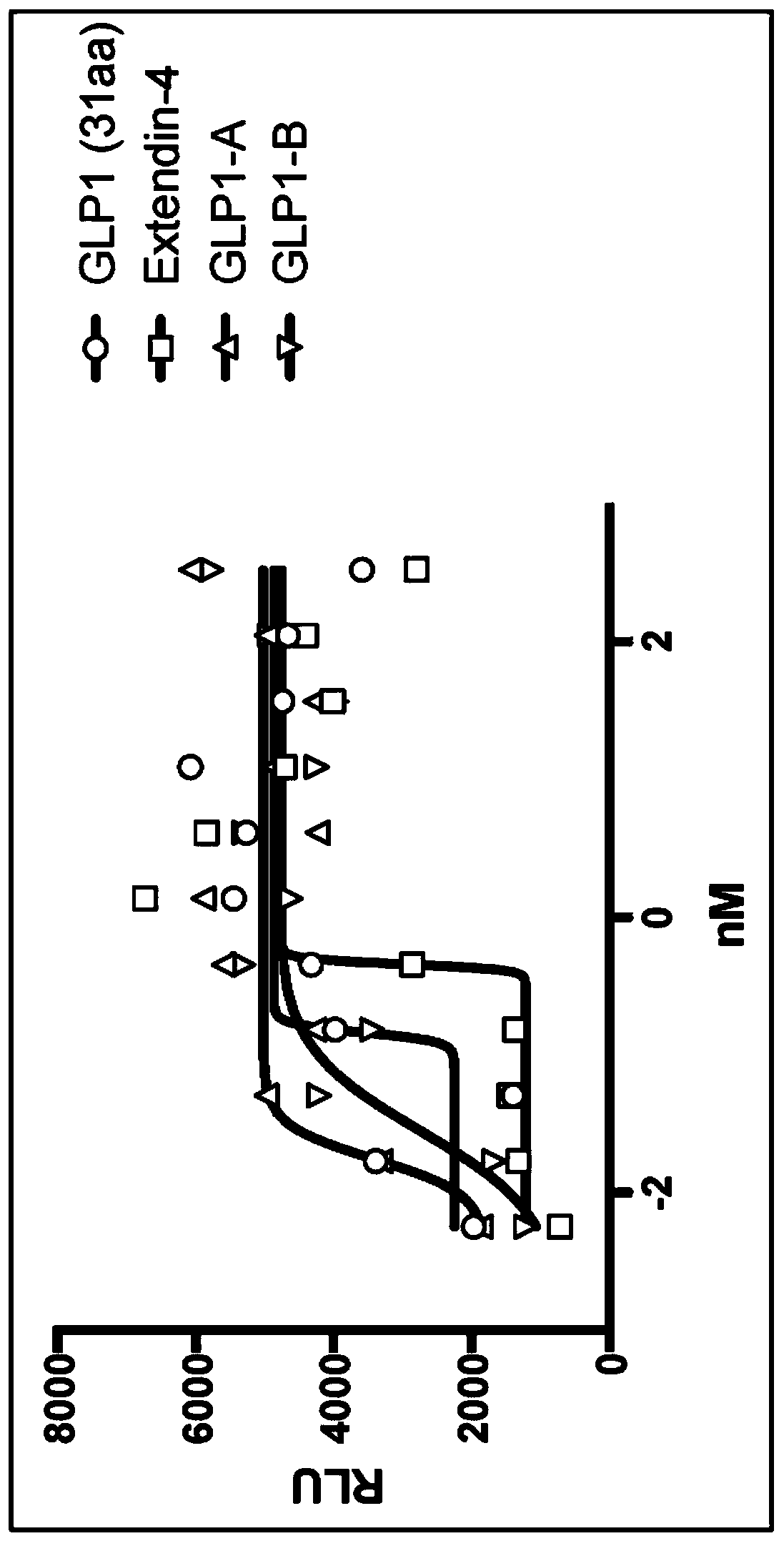
IgG Fc VARIANTS FOR VETERINARY USE
Provided are various embodiments relating to variant IgG Fc polypeptides of companion animals having increased Protein A binding for ease of purification, decreased C1q binding for reduced complement-mediated immune responses, decreased CD16 binding (e.g., for reduced antibody-dependent cellular cytotoxicity (ADCC) induction, increased stability, and / or the ability to form heterodimeric proteins.In addition, various embodiments relating to antibodies and fusion proteins comprising such variant IgG Fc polypeptides are provided. Also provided are various embodiments relating to contiguous polypeptides comprising one or more variant GLP1 polypeptide(s) having improved serum half-life. Further provided are various embodiments relating to contiguous polypeptides or heterodimeric polypeptides comprising a GLP1 polypeptide and a glucagon polypeptide as a dual GLP1 receptor and glucagon receptor agonist. In various embodiments, such polypeptides may be used to treat, for example, diabetes, obesity, or related indications, in companion animals, such as canines, felines, and equines.
Owner:KINDRED BIOSCI
Long-acting co-agonists of the glucagon and glp-1 receptors
ActiveUS20200270325A1Preventing and reducing in body weightNormalizing body fat distributionPeptide/protein ingredientsMetabolism disorderAgonistPancreatic glucagon
Owner:MERCK SHARP & DOHME LLC
Glucagon receptor modulators
Owner:PFIZER INC
Long-acting co-agonists of the glucagon and glp-1 receptors
ActiveUS20190338008A1Preventing and reducing in body weightNormalizing body fat distributionPeptide/protein ingredientsMetabolism disorderAgonistEphA Receptors
Owner:MERCK SHARP & DOHME LLC
Preparation method of glucagon
PendingCN111018963ATroubleshooting the connectionExtended reaction timePeptide preparation methodsBulk chemical productionPancreatic glucagonTetrapeptide
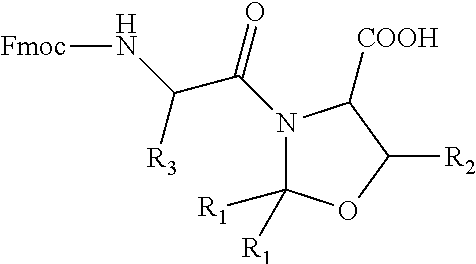
The invention relates to a preparation method for glucagon by a solid-phase fragment method. The method comprises the following steps: connecting 5-29 fragments one by one by utilizing a solid-phase synthesis method according to a sequence; and then connecting the tetrapeptide fragment Fmoc-His(Trt)-Ser(tBu)-Gln(Trt)-Gly-OH to 5-29 fragment peptide resin, carrying out acidolysis to remove a protecting group and cut peptide to obtain a glucagon crude product, and carrying out purification and freeze-drying to obtain a glucagon pure product. The method has the advantages of easiness in synthesis, low impurity content and cost saving.
Owner:CHINESE PEPTIDE CO
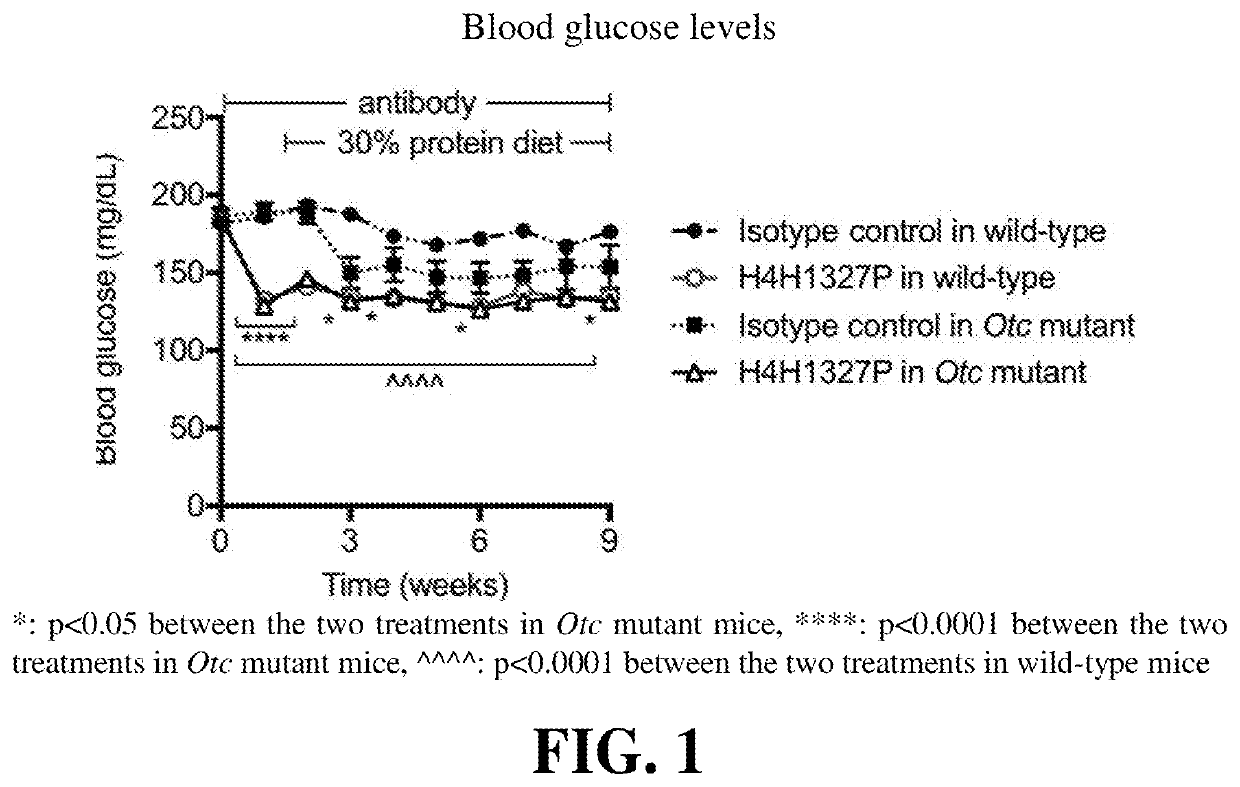
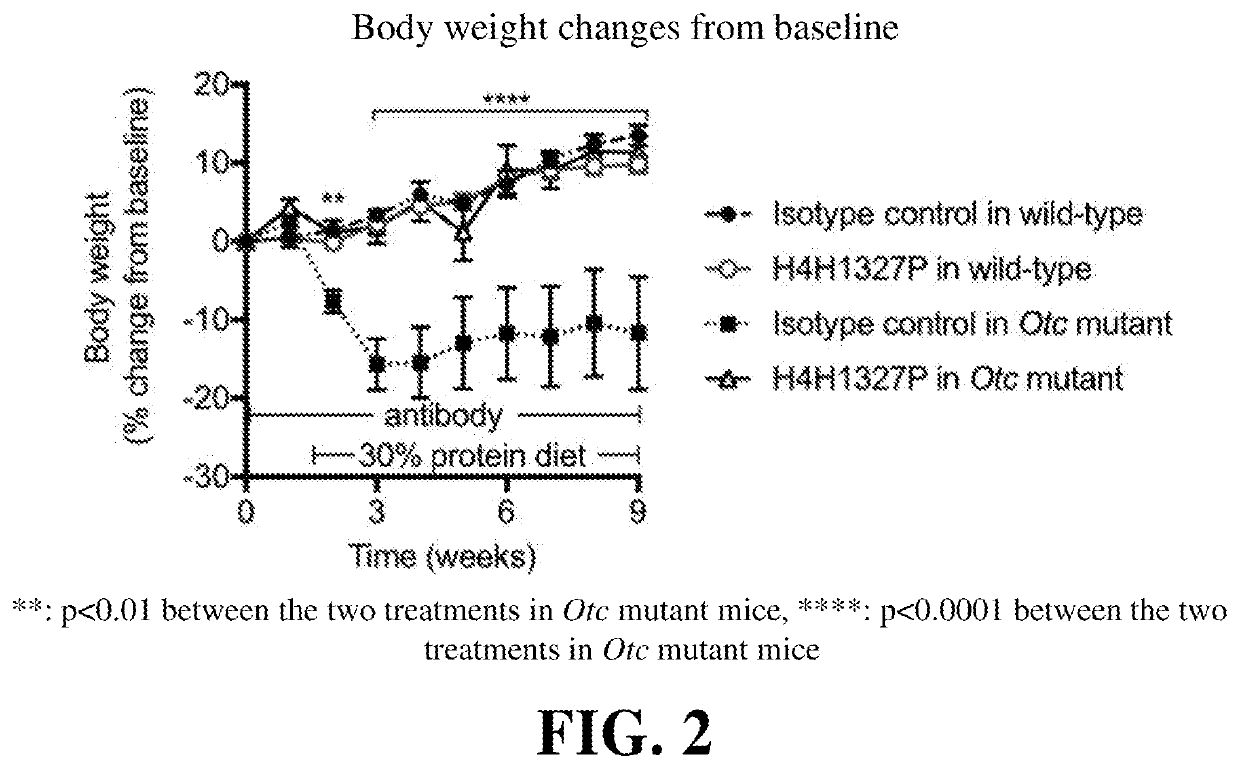
Methods of treating urea cycle disorders by interfering with glucagon receptor signaling
PendingUS20210130480A1Reduce the amount requiredInhibit bindingOrganic active ingredientsMetabolism disorderDiseaseAntiendomysial antibodies
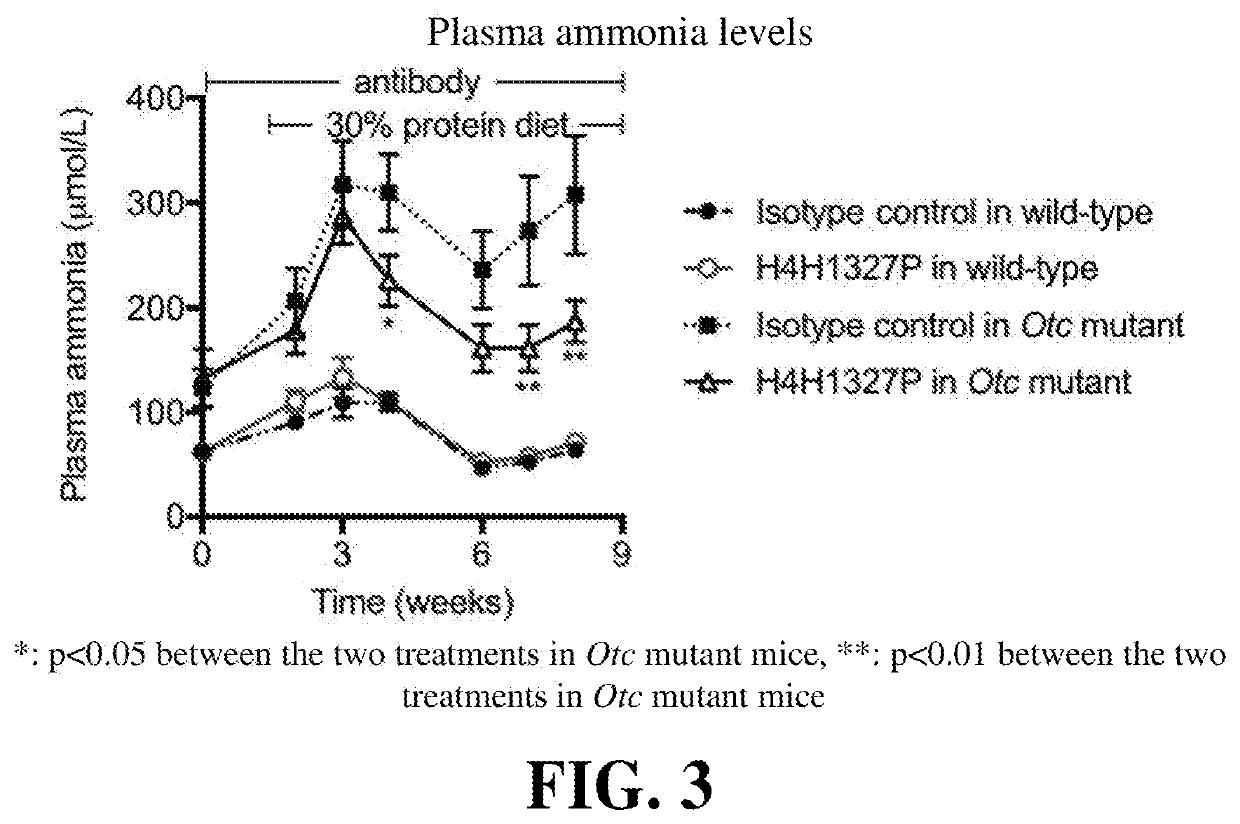
Provided herein are methods of treating a subject with hyperammonemia or a urea cycle disorder. The methods comprise administering to a subject in need thereof a therapeutic amount of a glucagon signaling pathway inhibitor, such that ammonia levels are lowered or that amino acid metabolism enzymes are down-regulated, or a condition or disease characterized by hyperammonemia is mediated, or at least one symptom or complication associated with the condition or disease is alleviated or reduced in severity. The glucagon signaling pathway inhibitor can be a small molecule inhibitor of the signaling pathway, an antisense inhibitor of the signaling pathway, shRNA, siRNA, a GCG neutralizing monoclonal antibody, a GCGR antagonist, a peptide inhibitor of the signaling pathway, a DARPin, a Spiegelmer, an aptamer, engineered Fn type-III domains, etc. The therapeutic methods are useful for treating a human suffering from hyperammonemia or a urea cycle disorder.
Owner:REGENERON PHARM INC
Exendin-4 derivatives as selective glucagon receptor agonists
ActiveCN106715466BImprove blood sugar levelsLose weightPeptide/protein ingredientsMetabolism disorderThiazoleAgonist
The present invention relates to glucagon receptor agonists and their medical use, for example for the treatment of severe hypoglycemia. Exendin-4 analogs are provided that potently and selectively activate the glucagon receptor and exhibit higher solubility at near neutral pH and enhanced in solution compared to native glucagon chemical stability. The analog has the artificial amino acid 4-thiazolealanine at position 1. This resulted in a higher selectivity for the glucagon receptor over the GLP1 receptor when compared to each other for the same compounds differing only in position 1 (Tza instead of His at position 1). The present invention provides highly selective glucagon receptor agonists.
Owner:SANOFI SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com