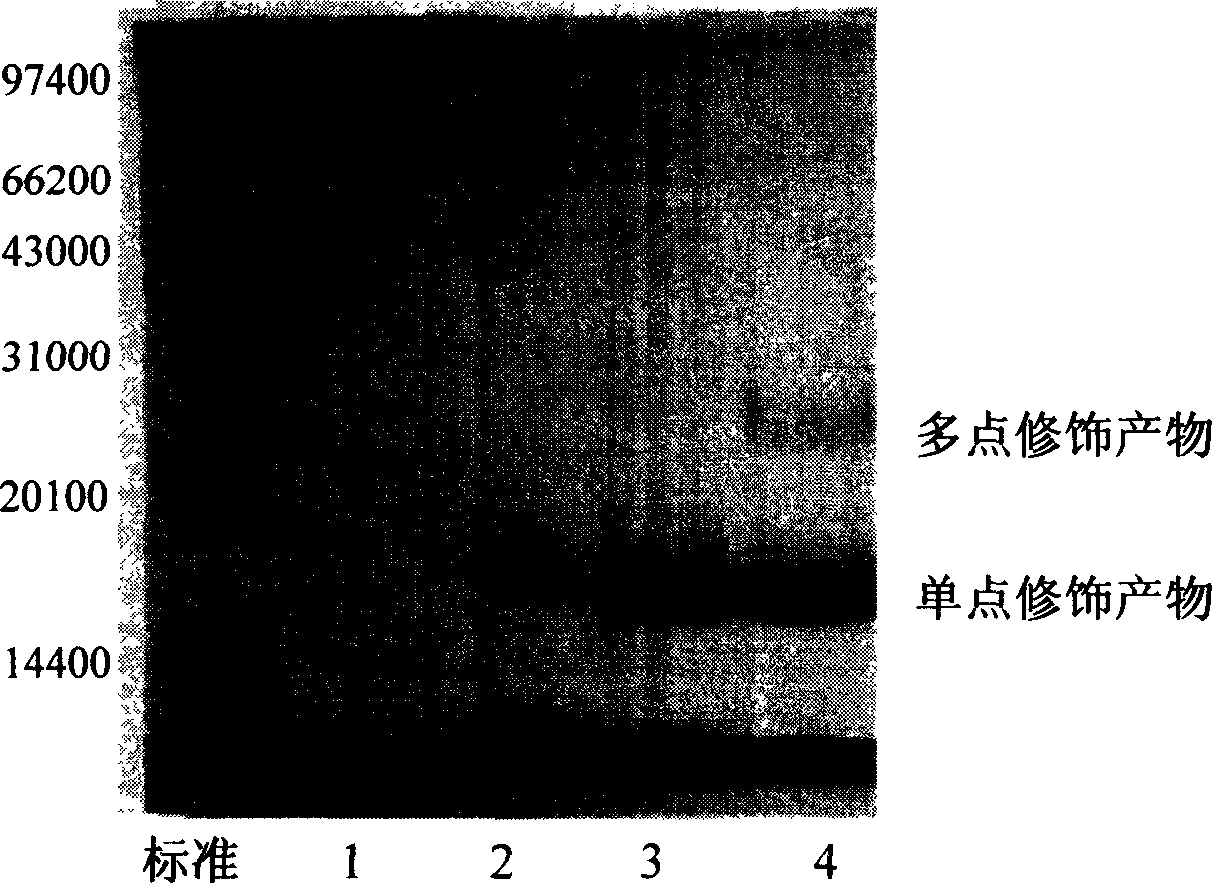
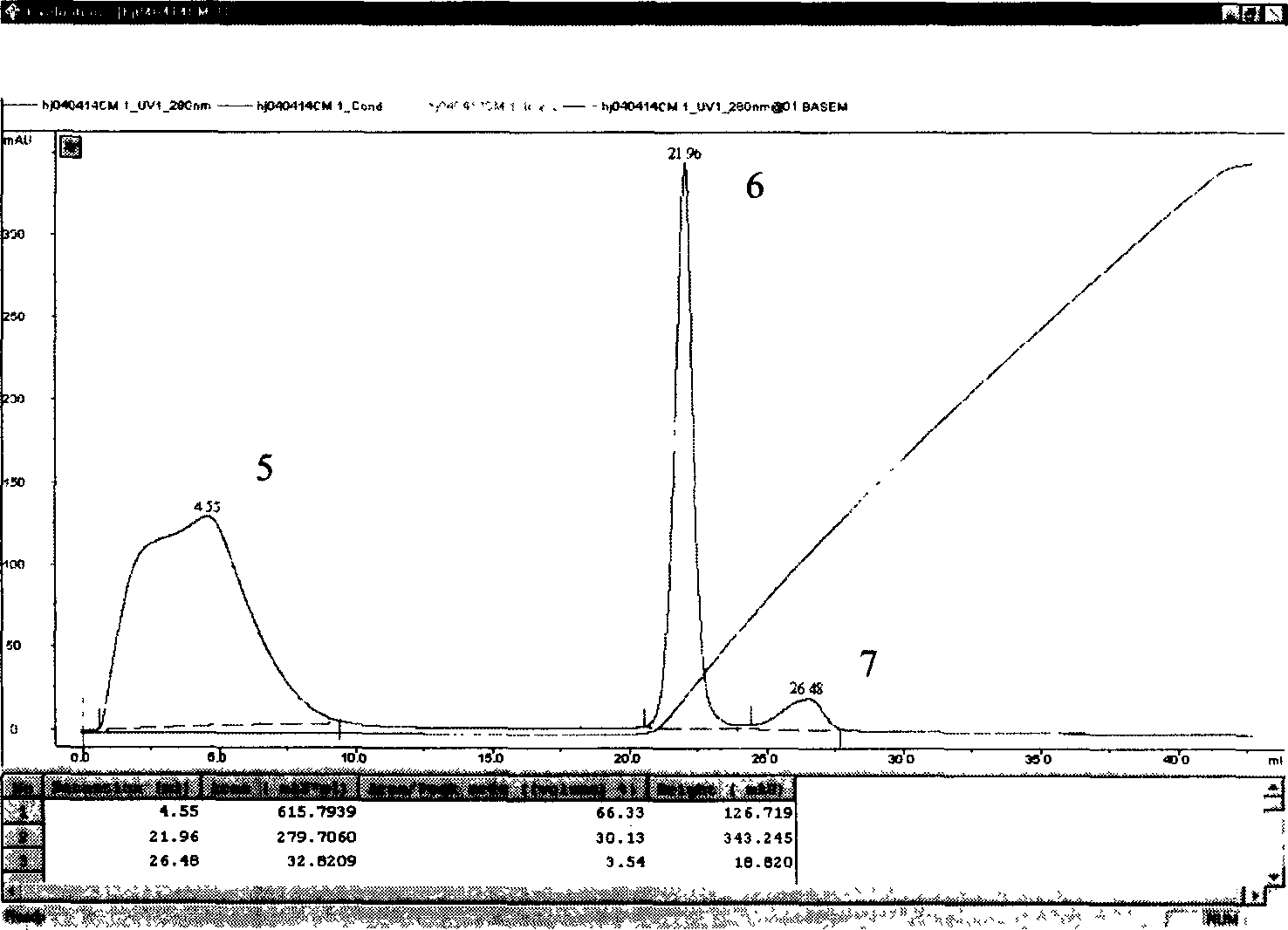
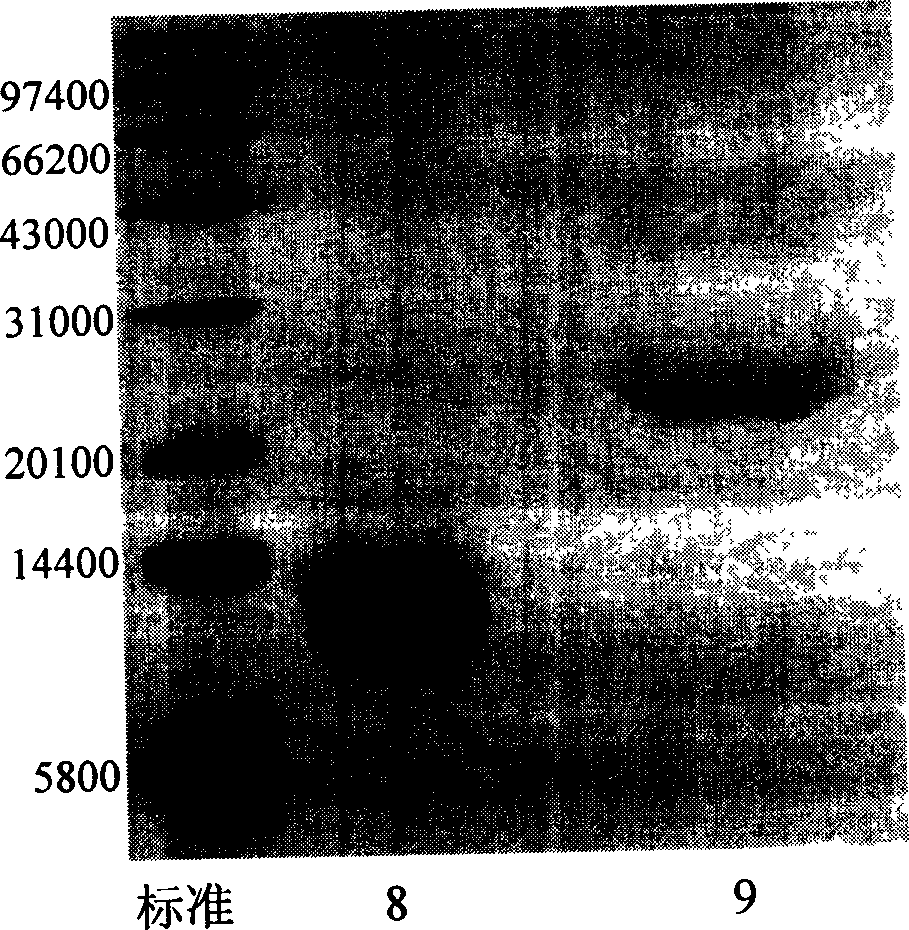
Human glucagon-like peptide-1 compound and its preparing method
A technology of glucagon and compound, which is applied in the field of peptide or protein modification compound and its preparation, which can solve the problems of poor biological stability, subcutaneous injection 3-4 times a day, and patients’ reluctance to accept it, and achieve production cost Low, improve bioavailability, improve the effect of biological stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of complexes of human glucagon-like peptide-1. In this embodiment, the active group of monomethoxypolyethylene glycol activated by succinimide is monomethoxypolyethylene glycol-succinimide propionate, ie mPEG-SPA, with a molecular weight of 5KD.
[0027] In the first step, 1.0mg of human glucagon-like peptide-1 (7-37) was dissolved in 0.5ml of 100mM, pH7.4 phosphate buffer, and then mixed with 0.5ml of 200mM phosphoric acid of pH6.0-9.0 Salt buffer or boric acid-borax buffer mixed;
[0028] In the second step, 1.5 mg of monomethoxypolyethylene glycol-propionic acid succinimide with a molecular weight of 5KD, i.e. mPEG-SPA, was added to the solution of the first step, and mixed uniformly;
[0029] In the third step, the solution obtained in the second step is placed at 4°C to 40°C for mPEG modification reaction for 0.5 to 24 hours;
[0030] In the fourth step, the solution treated in the third step is placed at -20°C to terminate the reaction, so fa...
Embodiment 2
[0037] Example 2 Preparation of complexes of human glucagon-like peptide-1. In this embodiment, the monomethoxypolyethylene glycol of the active group activated by succinimide is a branched monomethoxypolyethylene glycol-succinimide ester, namely mPEG-NHS, which The molecular weight is 10KD.
[0038] In the first step, 1.0 mg of human glucagon-like peptide-1 (7-37) was dissolved in 0.5 ml of 100 mM, pH 7.4 phosphate buffer, and then mixed with 0.5 ml of 200 mM phosphoric acid of pH 6.0 to 9.0 Salt buffer or boric acid-borax buffer mixed;
[0039] In the second step, 3.0 mg of branched monomethoxypolyethylene glycol-succinimide ester with a molecular weight of 10KD, i.e. mPEG-NHS, was added to the solution of the first step, and mixed uniformly;
[0040] The third step to the fifth step are carried out according to the corresponding operation steps in the first embodiment.
[0041] In the sixth step, the elution solution collected in the fifth step was concentrated with a Mi...
Embodiment 3
[0044] Example 3 Preparation of complexes of human glucagon-like peptide-1. In this embodiment, the monomethoxypolyethylene glycol of the active group activated by succinimide is monomethoxypolyethylene glycol-succinimide propionate, i.e. mPEG-SPA, and its molecular weight is 20KD.
[0045] In the first step, 1.0 mg of human glucagon-like peptide-1 (7-37) was dissolved in 0.5 ml of 100 mM pH7.4 phosphate buffer, and then mixed with 0.5 ml of 200 mM pH8.4 phosphate buffer solution or boric acid-borax buffer mixed;
[0046] In the second step, 6.0 mg of monomethoxypolyethylene glycol-propionic acid succinimide, i.e. mPEG-SPA, with a molecular weight of 20KD was added to the solution of the first step, and mixed uniformly;
[0047] The third step to the fifth step are carried out according to the corresponding operation steps in the first embodiment.
[0048] In the sixth step, the elution solution collected in the fifth step was concentrated with a MilliporeAmicon Ultra-15 ul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com