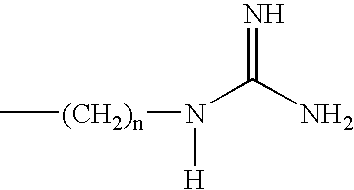
Methods and compositions for enhancing transport across biological membranes
a biological membrane and transport technology, applied in the direction of biocide, drug composition, peptide/protein ingredients, etc., can solve the problems of affecting the therapeutic utility of high-charge molecules, affecting the use of tat proteins, and affecting the transport of high-charge molecules. , to achieve the effect of promoting the transport of agents and enhancing the transport of selected compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0172] Peptide Synthesis
[0173] Peptides were synthesized using solid phase techniques on an Applied Biosystems Peptide synthesizer using FastMOC™ chemistry and commercially available Wang resins and Fmoc protected amino acids, according to methods well known in the art (Bonifaci et al., Aids 9:995-1000). Peptides were purified using C4 or C18 reverse phase HPLC columns, and their structures were confirmed using amino acid analysis and mass spectrometry.
example 2
[0174] Fluorescence Assays
[0175] Fluorescent peptides were synthesized by modification of the amino terminus of the peptide with aminocaproic acid followed by reaction with fluorescein isothiocyanate in the presence of (2-1H-benzotriazol-1-yl)-1,1,3,3-tetramethyl uronium hexafluorophosphate / N-hydroxy benzotriazole dissolved in N-methyl pyrrolidone. The products were purified by gel filtration.
[0176] Suspension cells (106 / mL) were incubated for varying times, at 37° C., 23° C., or 4° C., with a range of concentrations of peptides or conjugates in PBS pH 7.2 containing 2% fetal calf serum (PBS / FCS) in 96 well plates. After a 15 minute incubation, the cells were pelleted by centrifugation, washed three times with PBS / FCS containing 1% sodium azide, incubated with trypsin / EDTA (Gibco) at 37° C. for five minutes, then washed twice more with PBS / FCS / NaN3. The pelleted cells were resuspended in PBS containing 2% FCS and 0.1% propidium iodide and analyzed on a FACScan (Becton Dickinson, M...
example 3
[0177] Tat Basic Peptide Versus Poly-Arg Peptides
[0178] Uptake levels of the following polypeptides were measured by the method in Example 2: (1) a polypeptide comprising HIV tat residues 49-57 (Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg=SEQ ID NO: 1), (2) a nonamer of L-Lys residues (K9, SEQ ID NO:2), and (3) homo-L or homo-D-polypeptides containing four to nine Arg residues (SEQ ID NO:3-8 and 12-17). Results a re shown in FIGS. 1A-1C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com