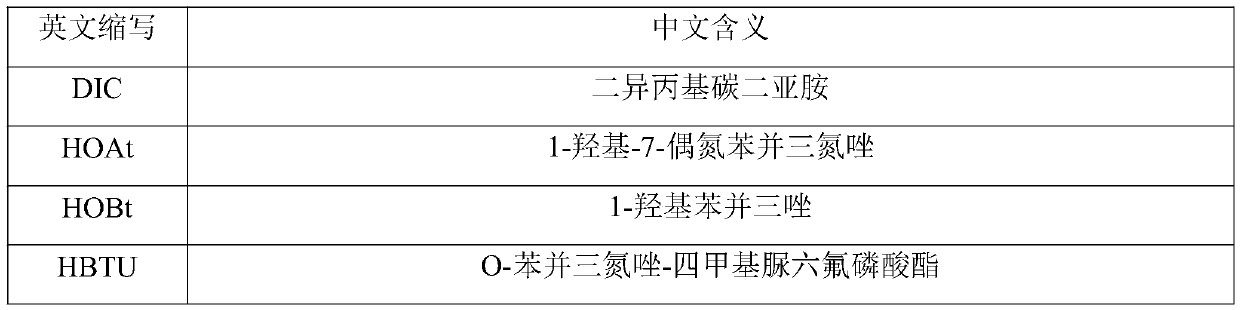
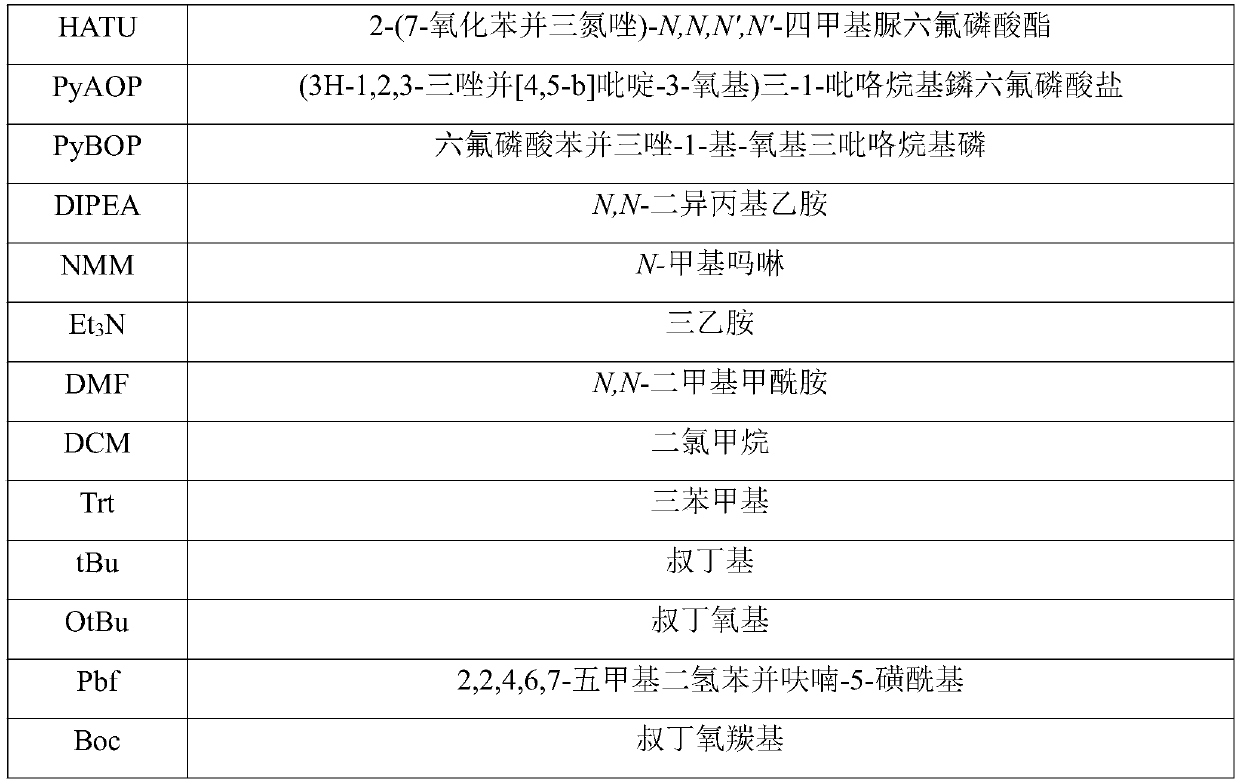
Preparation method of glucagon
A technology of glucagon and resin, which is applied in the field of polypeptide drug preparation, can solve the problems of low product purity, increased connection difficulty, high price, etc., and achieves the effects of reasonable reaction process, reduced side reactions, and fewer side reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Present embodiment adopts following technical scheme:
[0021] Step 1) Connect 5-29 fragments one by one according to the glucagon sequence by solid phase synthesis to obtain H-Thr(tBu)-Phe-Thr(tBu)-Ser(tBu)-Asp(OtBu)-Tyr(tBu) -Ser(tBu)-Lys(Boc)-Tyr(tBu)-Leu-Asp(OtBu)-Ser(tBu)-Arg(Pbf)-Arg(Pbf)-Ala-Gln(Trt)-Asp(OtBu)- Phe-Val-Gln(Trt)-Trp(Boc)-Leu-Met-Asn(Trt)-Thr(tBu)-Wang resin.
[0022] Specifically, the method for synthesizing the 5-29 fragment peptide resin in step 1) of the present invention is as follows: Fmoc-Thr(tBu)-OH is coupled with Wang resin to obtain Fmoc-Thr(tBu)-Wang resin. Wherein, the coupling reagent is 2,6-dichlorobenzoyl chloride and pyridine.
[0023] More specifically, Fmoc-Thr(tBu)-OH and Wang resin were mixed and dissolved in DCM, and pyridine was added to react with 2,6-dichlorobenzoyl chloride.
[0024] Preferably, the molar ratio of Wang resin to Fmoc-Thr(tBu)-OH, 2,6-dichlorobenzoyl chloride and pyridine is 1:1.2:2.4:4.8.
[0025] Prefe...
example 1
[0043] Example 1: the preparation of the Fmoc-Thr(tBu)-Wang resin that the degree of substitution is 0.23mmol / g
[0044] Weigh 50.00g of blank Wang resin with a substitution degree of 0.79mmol / g, Fmoc-Thr(tBu)-OH 9.41g, mix and add DCM to dissolve, then add 7.65mL of 4 equivalents of pyridine to amino acids, and finally slowly add 2,6 -Dichlorobenzoyl chloride 6.78mL, stirred and reacted for 3h, transferred to the reaction column, pumped off the reaction solution, washed 3 times with DMF, 1 time with methanol, 3 times with DMF and 3 times with methanol, and dried the solvent After vacuum drying, Fmoc-Thr(tBu)-Wang resin was obtained, and the detected substitution degree was 0.23 mmol / g.
example 2
[0045] Example 2: the preparation of Fmoc-Thr(tBu)-Wang resin with a degree of substitution of 0.41mmol / g
[0046] Weigh 50.00g of blank Wang resin with a substitution degree of 0.79mmol / g, Fmoc-Thr(tBu)-OH 18.83g, mix and add DCM to dissolve, then add 15.3mL of 4 equivalents of pyridine to amino acids, and finally slowly add 2,6 -Dichlorobenzoyl chloride 13.56mL, after stirring for 3 hours, transfer to the reaction column, remove the reaction liquid, wash 3 times with DMF, wash 1 time with methanol, wash 3 times with DMF, wash 3 times with methanol, and drain the solvent After vacuum drying, Fmoc-Thr(tBu)-Wang resin was obtained, and the detected substitution degree was 0.41 mmol / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com