Recombinant human serum albumin/pancreatic glucagon peptide fusion protein having blood sugar content continuous control function
A serum albumin and fusion protein technology, applied in the field of long-acting medicinal functions, can solve the problems of limiting the clinical application of GLP-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Brief Description of Molecular Cloning Technology
[0077] Conventional molecular cloning techniques include extraction of DNA and RNA, agarose gel and polyacrylamide gel electrophoresis, connection of DNA fragments, and restriction endonuclease digestion reactions are all referred to literature (Maniatis, et al., "Molecular cloning experiment Handbook" published by Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, 1982). The purification of plasmid DNA and the recovery of DNA fragments are all prepared by commercial purification columns. DNA polymerase chain reaction (PCR) (referring to literature Saiki et al., Science, 230: 1350, 1985) used enzymes and PCR instruments required for the reaction are all Perkin Elmer products. And refer to the manufacturer's operating procedures. The oligonucleotide primers required for DNA sequencing and DNA amplification are completed by specialized institutions. The transgenic Escherichia coli was purchased fr...
Embodiment 2
[0078] Embodiment 2: Human serum albumin (HSA) gene expression and the construction of carrier plasmid
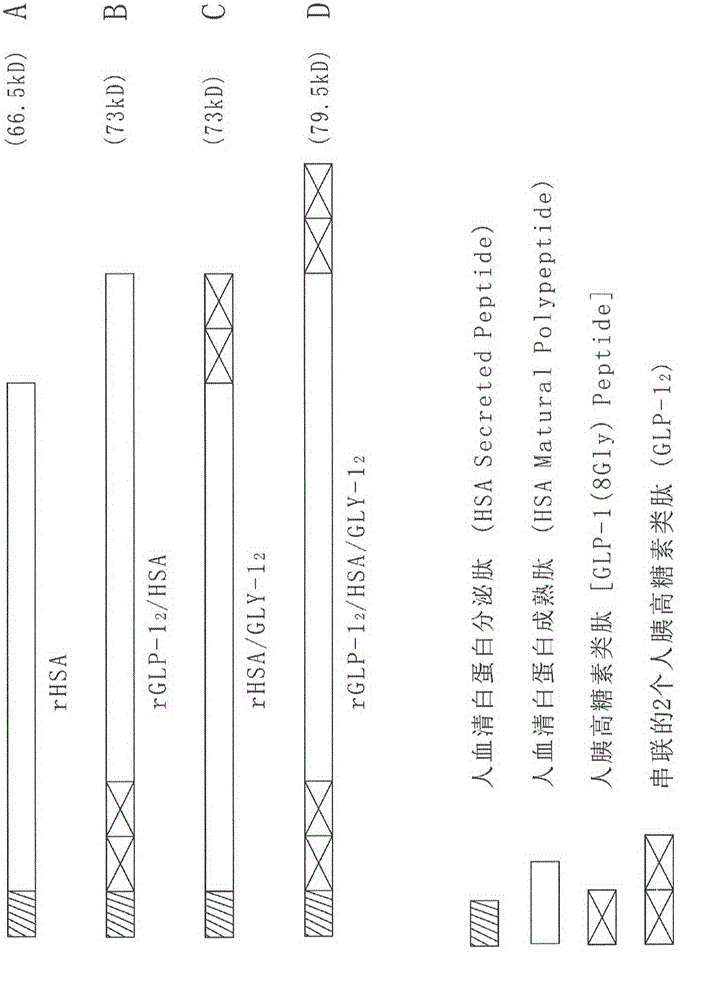
[0079] The HSA cloning method and test results described by the inventor in its invention patents ZL02142881.6, ZL200410042814.8 and ZL200410057313.7 were used. The HSA sequence of GenBank retrieval number AY728024 is used for gene recombination fusion protein of HSA gene sequence and cell growth factor gene sequence in the present invention. Using AY728024 sequence to encode HSA can make the fusion protein obtain unexpected high-level expression in yeast. Expression plasmids (see Chinese patent ZL02142881.6) can also be used in the embodiments of the present invention. The recombinant human serum albumin expression vector plasmid DNA structure of pYZ-HSA is also in the Chinese invention patent ZL02142881.6 figure 2 Disclosed in and used to construct various molecular structure expression vector basic plasmids of the recombinant human serum albumin / glucagon peptide fusio...
Embodiment 3
[0080] Example 3: Synthesis of Human GLP-1 Tandem Double Gene Fragment and Recombinant Human Serum Albumin / Glucagon Peptide 2 Construction of Fusion Protein Expression Vector Plasmid
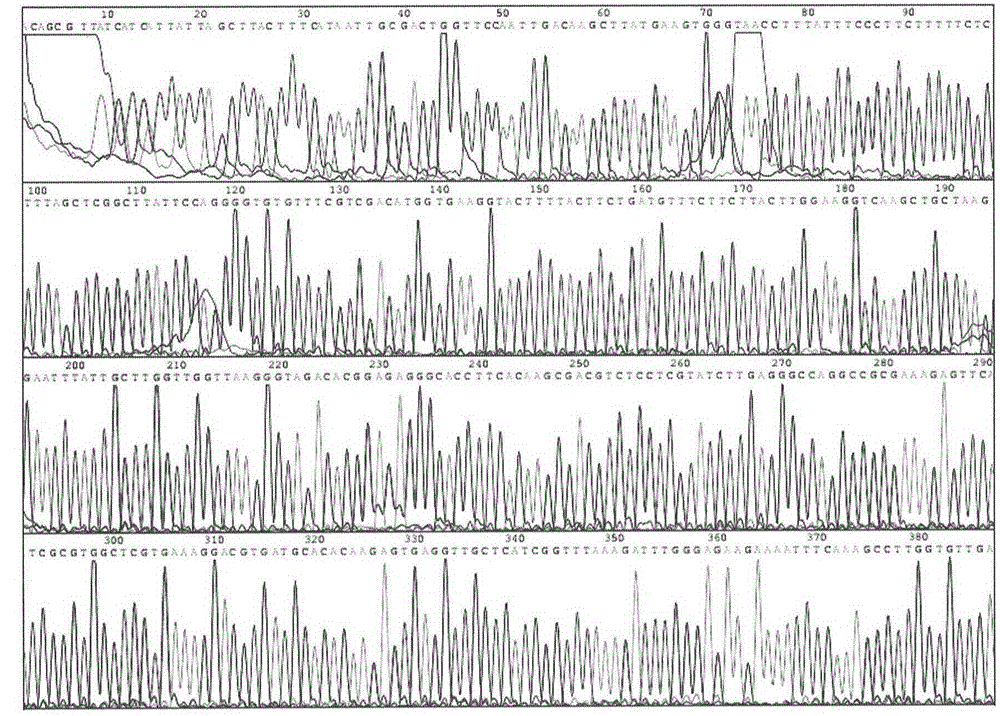
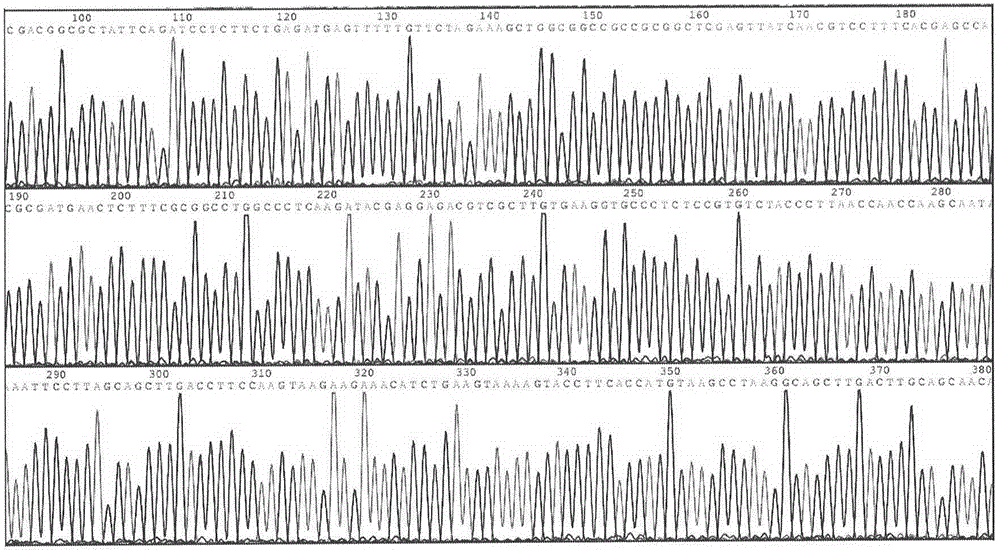
[0081] The inventor first synthesized the GLP-1 gene sequence using the GLP-1 wild-type amino acid sequence 7-36 peptide as a standard, and fused it with the human serum albumin gene to obtain various GLP-1 fusion proteins and carried out biological activity verification. Then, using the GLP-1 analogue amino acid sequence in Albugon of HGSI in the United States as a standard, the DNA sequence total synthesis method was used to artificially synthesize the double repeat sequence of human GLP-1 (7-36, Ala8Gly) as Seq No.3, corresponding to The amino acid sequence of is Seq No.4. The synthesized GLP-1 2 The N-terminal is equipped with a Bsu36I endonuclease site to achieve seamless connection and direct connection with the C-terminal of human serum albumin:
[0082] Seq No.3:
[0083] 5'-G CTTA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com