Multiple inducible gene regulation system
a gene regulation and inducible technology, applied in the field of biotechnology or genetic engineering, can solve the problems of limited gene regulation at the moment, significant qualitative and quantitative limitations, and inability to regulate more than one gene at the same time in the same cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
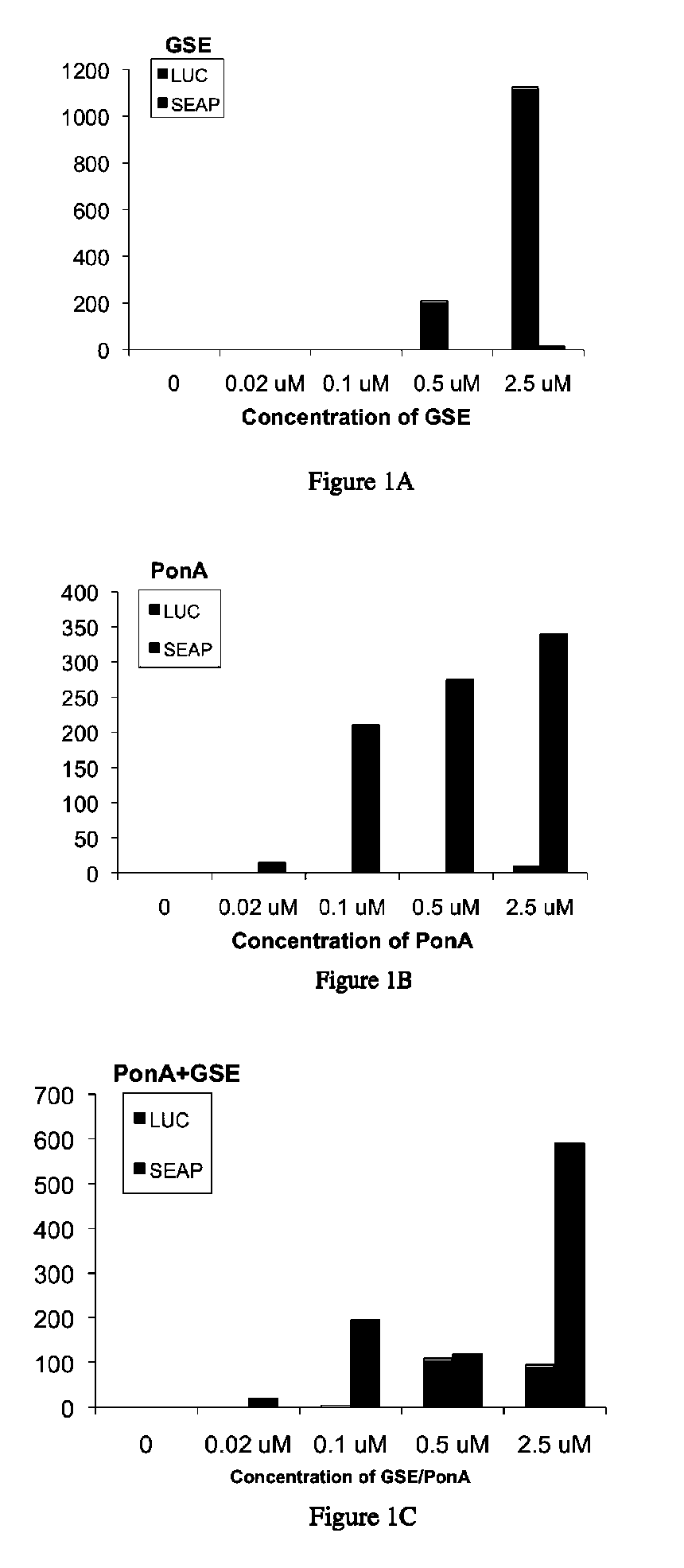
[0277]This Example describes the construction of several gene expression cassettes for use in a multiple inducible gene expression system according to the invention. Applicants constructed several gene expression cassettes based on the spruce budworm Choristoneura fumiferana EcR (“CfEcR”), fruit fly Drosophila melanogaster EcR (“DmEcR”), green leafhopper Nephotetix cincticeps ecdysone receptor (“NeEcR”), mouse Mus musculus retinoid X receptor isoform α (“MmRXRα”), and locust Locusta migratoria invertebrate RXR homolog ultraspiracle protein (“LmUSP”). The prepared receptor constructs comprise a ligand binding domain of either an EcR or a vertebrate RXR; and a GAL4 DNA binding domain (DBD) or a VP16 transactivation domain (AD). The reporter constructs include a reporter gene, luciferase (Luc) or secreted alkaline phosphatase (SEAP), operably linked to a synthetic promoter construct that comprises a GALA response element to which the GAL4 DBD binds. Various combinations of these recept...
example 2
[0279]This Example describes the ability of a dual switch gene expression system of Applicants' invention to modulate expression of two reporter gene expression cassettes, wherein the two reporter gene expression cassettes are regulated independently by two different ligands. Specifically, one reporter gene expression cassette is inducibly regulated by a steroid ligand and the other reporter gene expression cassette is inducibly regulated by a non-steroid ligand. Briefly, Applicants prepared a dual switch inducible gene expression system as described above in Example 1.1. The resulting dual switch system was then tested in NIH3T3 mammalian cells as follows.
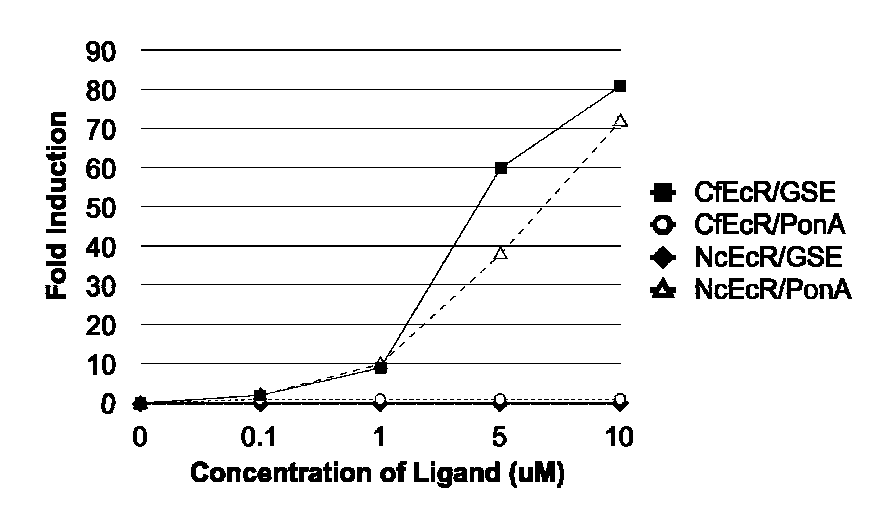
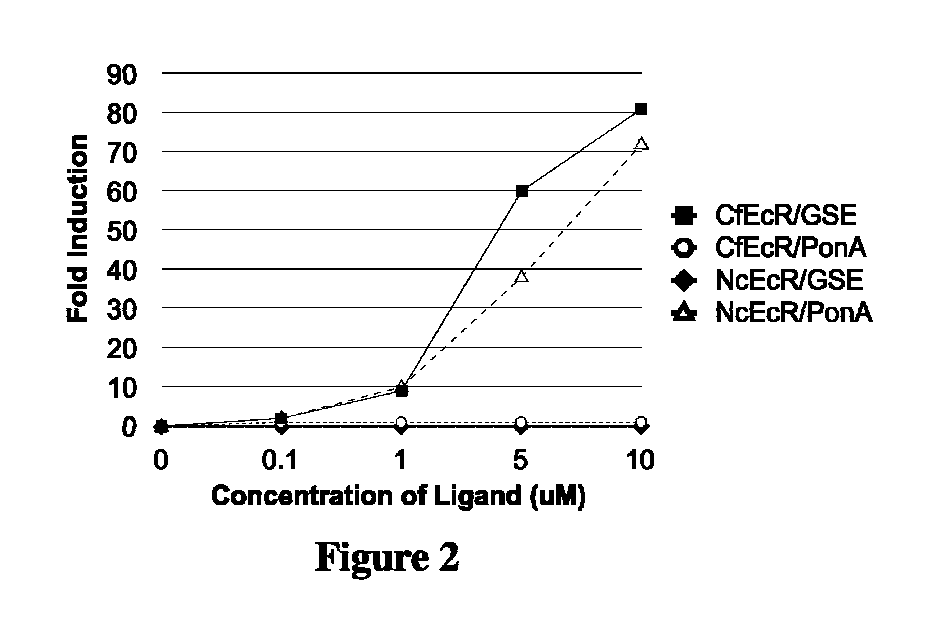
[0280]DNAs corresponding to the dual switch constructs outlined in Example 1.1 were transfected into mouse NIH3T3 cells (ATCC) as described in Example 1. At the end of the transfection incubation period, 250 μl of growth medium containing 20% FBS and either dimethylsulfoxide (DMSO; control) or a DMSO solution of 0.02, 0.1, 0.5, or...
example 3
[0282]This Example describes the ability of a dual switch gene expression system of Applicants' invention to modulate expression of two reporter gene expression cassettes, wherein the two reporter gene expression cassettes are regulated independently by two different ligands. In particular, one reporter gene expression cassette is inducibly regulated by a steroid ligand and the other reporter gene expression cassette is inducibly regulated by a non-steroid ligand. Briefly, Applicants prepared a dual switch inducible gene expression system as described above in Example 1.2. The resulting dual switch system was then tested in Chinese hamster ovary CHO cells as follows.
[0283]DNAs corresponding to the dual switch constructs outlined in Example 1.2 were transfected into hamster CHO cells (ATCC) as described in Example 1. CHO cells were transfected with 1) GAL4CfEcR-DEF / VP16MmRXRα-EF and pFRLuc, or 2) GAL4NcEcR-CDE / VP16MmRXRα-EF and pFRLuc. At the end of the transfection incubation period...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com