IgG Fc VARIANTS FOR VETERINARY USE
A variant, amino acid technology, applied in the introduction of foreign genetic material, antibodies, drug combinations, etc. using vectors, can solve the problems of weak CD16 affinity, inability to measure, and weak C1q affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
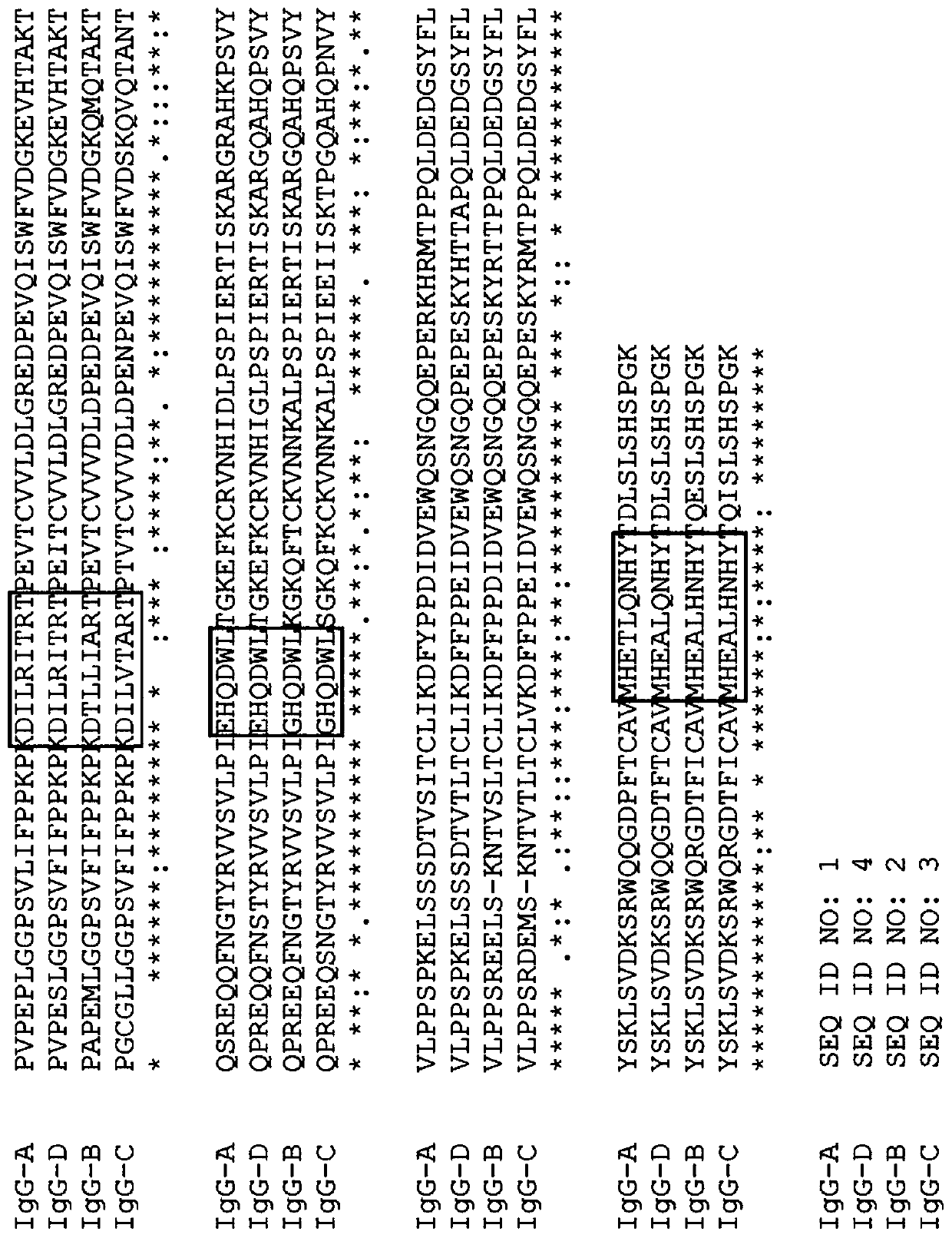
[0474] Variant canine IgG Fc polypeptides with increased protein A binding and / or decreased complement binding and / or decreased CD16 binding
[0475] Purification of antibodies using protein A affinity is a well-established process. However, of the four subtypes of canine IgG, only IgG-B Fc (eg, SEQ ID NO: 2 or SEQ ID NO: 107) has protein A binding affinity. Canine IgG-A Fc (eg, SEQ ID NO: 1), IgG-C Fc (eg, SEQ ID NO: 3 or SEQ ID NO: 108), and IgG-D Fc (eg, SEQ ID NO: 4) have weak or no Measured protein A binding affinity. Variant canine IgG-A Fc, IgG-C Fc and IgG-D Fc polypeptides were designed to alter Protein A binding.
[0476] Furthermore, canine IgG-B Fc and IgG-C Fc have complement activity and bind to C1q, while canine IgG-A Fc and IgG-D Fc have weak or unmeasurable binding affinity for C1q. To potentially reduce C1q binding and / or potentially reduce complement-mediated immune responses, variant canine IgG-B Fc and IgG-C Fc polypeptides were designed.
[0477] In a...
Embodiment 2
[0497] Variant Equine IgG Fc Polypeptides with Increased Protein A Binding and / or Reduced Complement Binding
[0498] Of the seven subtypes of equine IgG, IgG1 Fc (eg, SEQ ID NO: 63), IgG3 Fc (eg, SEQ ID NO: 65), IgG4 Fc (eg, SEQ ID NO: 66), IgG7 Fc (eg, SEQ ID NO: 66), IgG7 Fc (eg, SEQ ID NO: 63) : 69) with protein A binding affinity. Equine IgG2 Fc (eg, SEQ ID NO: 18, SEQ ID NO: 64), IgG5 Fc (eg, SEQ ID NO: 67), and IgG6 Fc (eg, SEQ ID NO: 68) have weak or unmeasurable protein A binding Affinity. Variant equine IgG2 Fc, IgG5 Fc and IgG6 Fc polypeptides were designed to alter protein A binding.
[0499] Furthermore, equine IgG2 Fc, IgG5 Fc, and IgG6 Fc have weak or unmeasurable binding affinity for C1q, while equine IgGl Fc, IgG3 Fc, IgG4 Fc, and IgG7 Fc bind to C1q. Variant equine IgGl Fc, IgG3 Fc, IgG4 Fc and IgG7 Fc polypeptides were designed to potentially reduce C1q binding and / or potentially reduce complement-mediated immune responses.
[0500] Table 6 below summari...
Embodiment 3
[0512] Variant feline IgG Fc polypeptides with reduced complement fixation
[0513]Each of the three subtypes of feline IgG (IgG1a Fc (SEQ ID NO: 80 or SEQ ID NO: 117), IgG1b Fc (SEQ ID NO: 81 or SEQ ID NO: 118) and IgG2 Fc (SEQ ID NO: 118) ID NO: 16)) all have protein A binding affinity. However, only feline IgG2 Fc had weak or unmeasurable binding affinity for C1q, while feline IgG1aFc, IgG1b Fc bound to C1q. To potentially reduce C1q binding and / or potentially reduce complement-mediated immune responses, variant feline IgG1a Fc and IgG1b Fc polypeptides were designed.
[0514] Table 8 below summarizes the protein A and C1q binding properties of feline IgG Fc subtypes. It should be noted that none of the wild-type feline IgG Fc subtypes lacked binding to C1q and binding to protein A.
[0515] Table 8
[0516]
[0517] (–) indicates low or unmeasurable binding activity.
[0518] To potentially reduce the binding of C1q to feline IgG1a Fc and IgG1b Fc, and / or potential...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com