Novel stable preparation of recombinant human pancreatic glucagon-like peptide-1 analogue fusion protein
A technology of glucagon and fusion protein, applied in the field of new pharmaceutical preparations, can solve the problems of decreased protein activity, decreased therapeutic effect, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of recombinant human glucagon-like peptide-1 analogue fusion protein: the strain is inoculated in YPD medium, shaken at 220-280rpm at 30°C until the wet weight of the bacteria is about 50g / L, put into the tank according to 10% inoculation amount, and culture After 20 hours, the induction was started by continuous feeding of methanol. After 4 hours of induction, the temperature was lowered to 22°C. After 50 hours, the induction was terminated, and the fermentation supernatant was collected by centrifugation at 10000 g for 15 minutes. Purification adopts four-step chromatography of BLUE affinity, PHE hydrophobicity, DEAE ion exchange and gel exclusion. Firstly, the fermentation supernatant was diluted 3 times with 20mmol / L pH7.0 sodium phosphate solution, passed through the Blue Sepharose Fast Flow affinity chromatography column with 2M NaCl, 20mmol / L pH6.5 sodium phosphate solution to elute the target protein. Add (NH4) to the collected protein solution 2 SO...
Embodiment 2
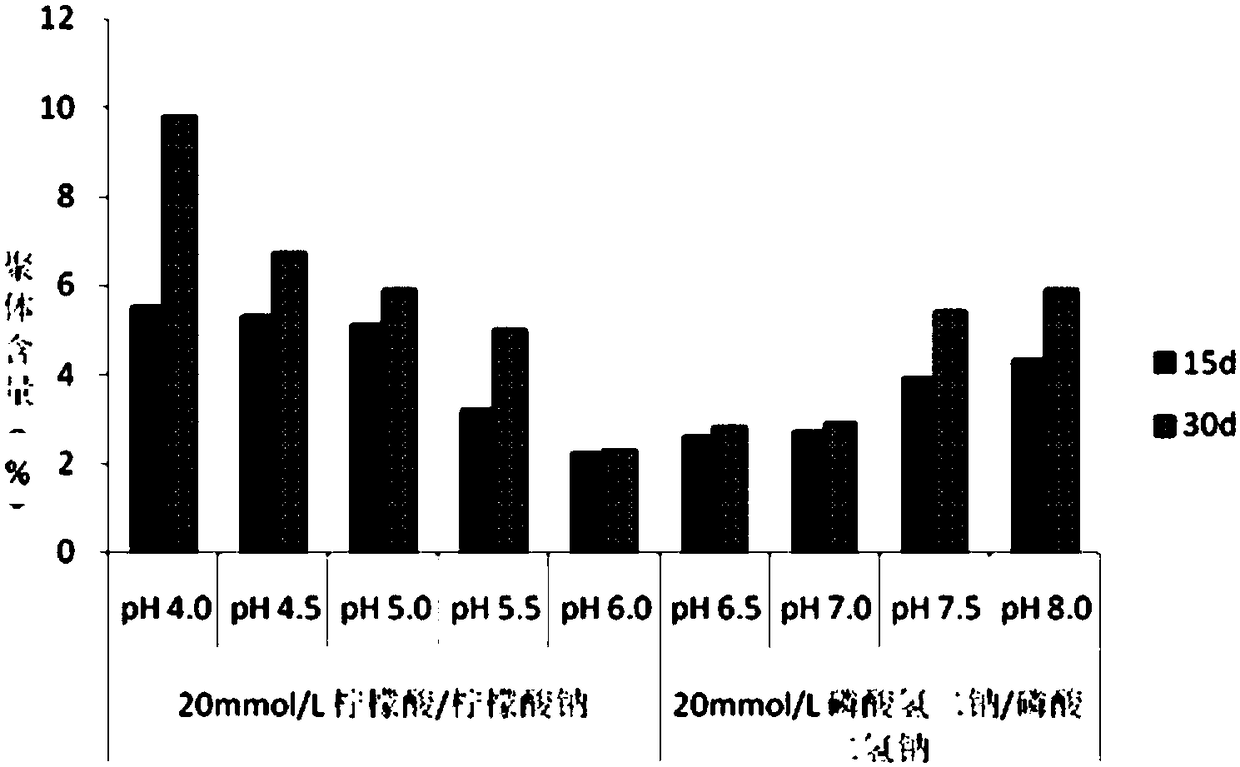
[0031] The effect of pH on the stability of recombinant human glucagon-like peptide-1 analogue fusion protein composition was investigated.
[0032] The purity of recombinant human glucagon-like peptide-1 analogue fusion protein was evaluated by size exclusion HPLC. Samples were analyzed using a size exclusion column (7.8mm*30cm) with an isocratic mobile phase consisting of phosphate and sulfate. Purity was determined by calculating the ratio of the main peak area to the total peak area at a detection wavelength of 214 nm. Each group contained recombinant human glucagon-like peptide-1 analogue fusion protein 1.0mg / ml, mannitol 30mg / ml, histidine 10mg / ml, polysorbate 80 1mg / ml, 20mmol / L of different pH Citric acid / sodium citrate buffer or disodium hydrogen phosphate / sodium dihydrogen phosphate buffer, pH 4.0 to pH 8.0, with intervals of 0.5 between groups. The samples of each group were sterile filtered and distributed into sterile pyrogen-free vials. Accelerated investigati...
Embodiment 3
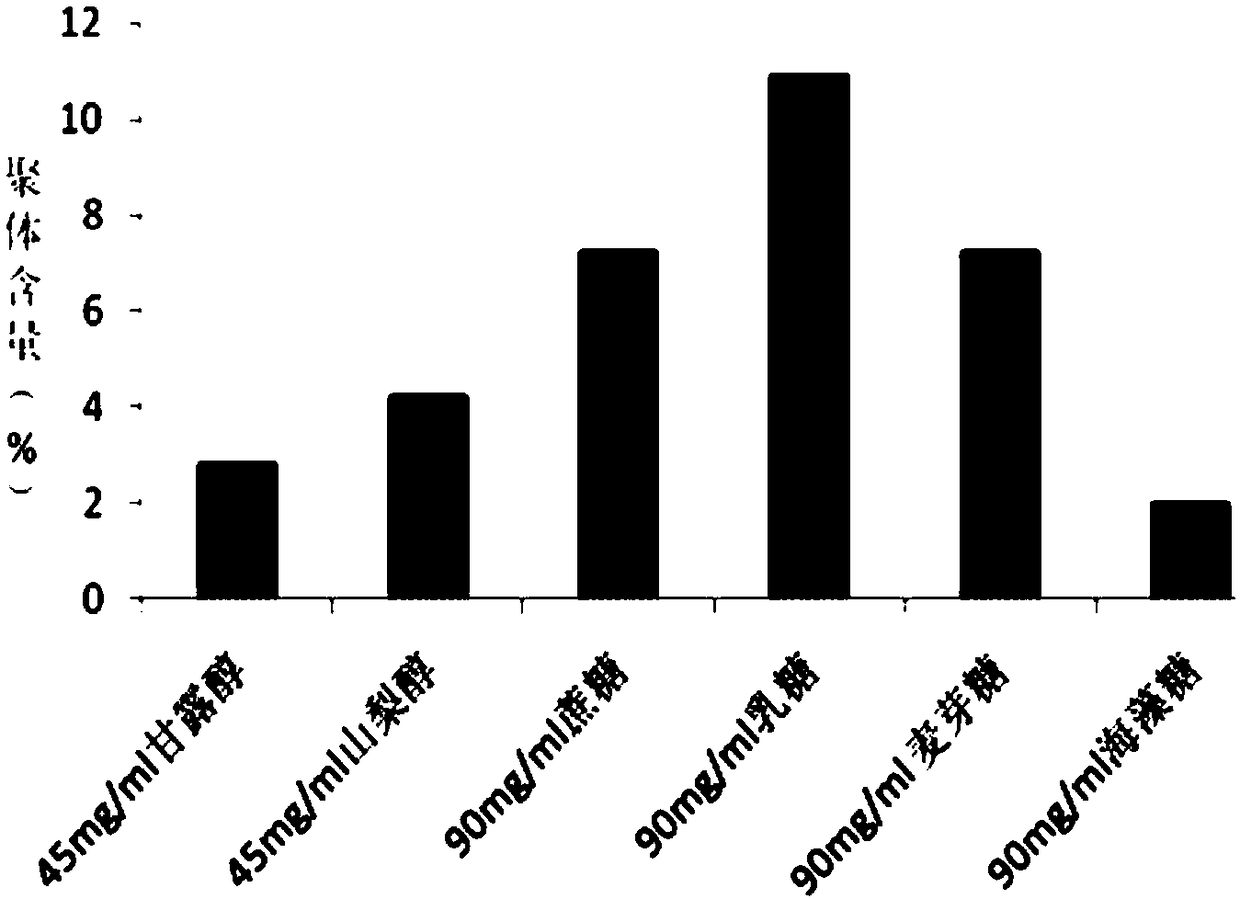
[0037] The selection of embodiment 3 excipients
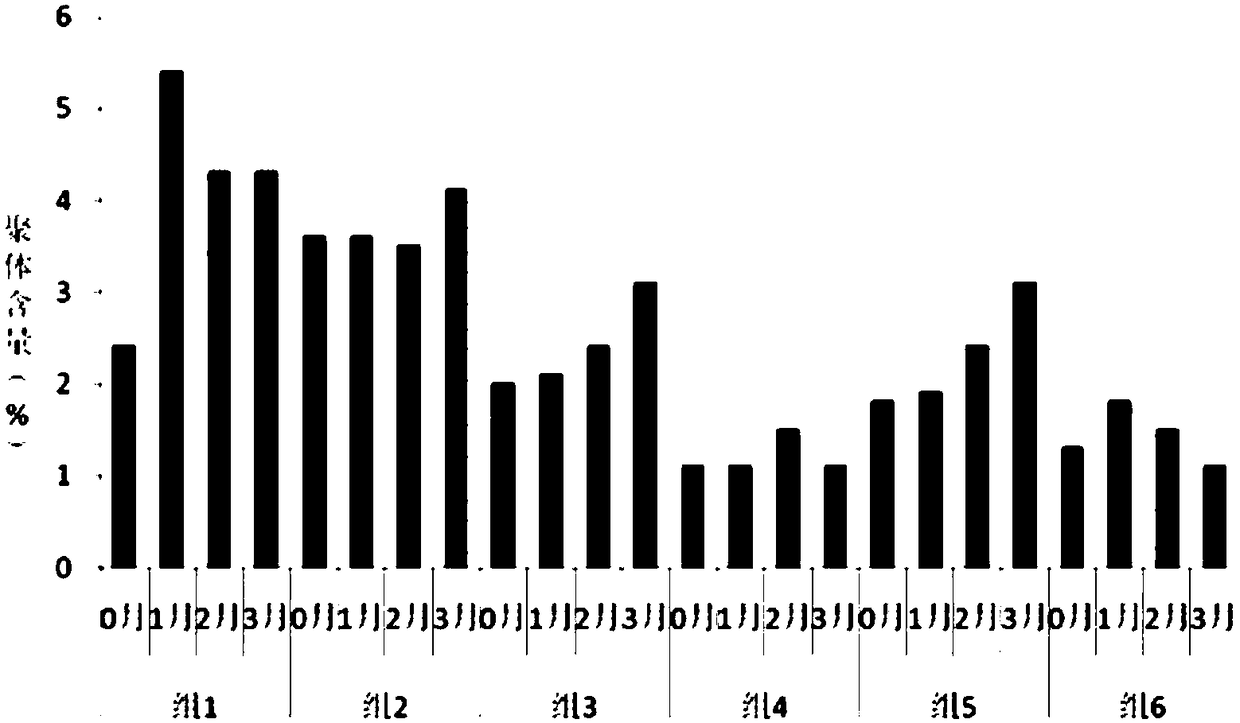
[0038] Some excipients and auxiliary materials suitable for human use were screened, and their effects on the formation of recombinant human glucagon-like peptide-1 analogue fusion protein preparations during freeze-drying were studied. In each group, the recombinant human glucagon-like peptide-1 analogue fusion protein solution was ultrafiltered into 20mmol / L pH 6.5 phosphate buffer, the protein concentration was 50.0mg / ml, and various excipients, types and The concentration is detailed in Table 2. After the sample solution was filtered and sterilized, it was divided into sterile vials with a volume of 1.0ml, half stoppered, and freeze-dried. The lyophilized products were visually inspected, and protein aggregates were detected by SEC-HPLC.
[0039] Table 2 Excipient results and SEC-HPLC results of several excipients after freeze-drying
[0040]
[0041] The results show that, from the excipient effect, mannitol is used ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com