Glucagon analogue, and preparation method and application thereof
A technique for glucagon and analogues, applied in the field of glucagon analogues and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The fifth aspect of the present invention provides the preparation method of the glucagon analog provided in the first aspect of the present invention, and the preparation method may include: preparing the glucagon analog by chemical synthesis; the preparation method also It may include: cultivating the host cell provided by the fourth aspect of the present invention under appropriate conditions to express the glucagon-like polypeptide fragment, isolating and purifying the glucagon-like polypeptide fragment, and then extracting the A long-acting carrier is chemically cross-linked to the glucagon-like polypeptide fragment. The glucagon analogs of the present invention can be prepared by standard peptide synthesis methods, e.g., by standard solid-phase or liquid-phase methods, stepwise or by fragment assembly, and isolation and purification of the final glucagon-like polypeptide fragments, pancreatic Glucagon analog products, or any combination by recombinant and syntheti...
Embodiment 1
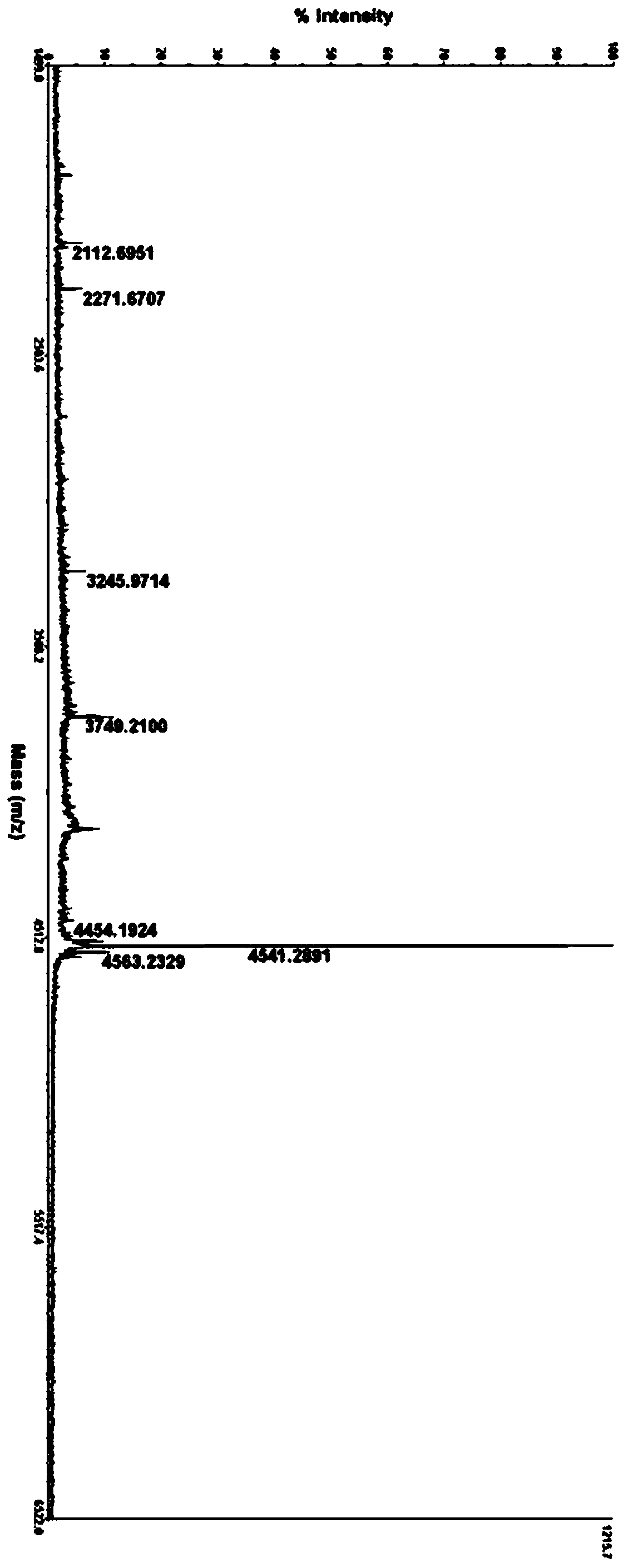
[0122] Preparation of glucagon derivative FC382K14D21:
[0123]
[0124] HSQGT FTSDY SKYXD SQAAQ DFVQW LMNGG PSSGA PPPS-OH
[0125] X14=K(palmitoyl-γE) (SEQ ID NO.16)
[0126] Fmoc-protected amino acids were purchased from Chengdu Zhengyuan Biochemical Technology Co., Ltd., and the following amino acids were used during the extended synthesis of polypeptides: Fmoc-L-Ala-OH, Fmoc-L-Asn(Trt)-OH, Fmoc-L-Asp( OtBu)-OH, Fmoc-L-Cys(Trt)-OH, Fmoc-L-Gln(Trt)-OH, Fmoc-L-Glu(OtBu)-OH, Fmoc-Gly-OH, Fmoc-L-His( Trt)-OH, Fmoc-L-Ile-OH, Fmoc-L-Leu-OH, Fmoc-L-Lys(Boc)-OH, Fmoc-L-Met-OH, Fmoc-L-Phe-OH, Fmoc- L-Pro-OH, Fmoc-L-Ser(tBu)-OH, Fmoc-L-Thr(tBu)-OH, Fmoc-L-Trp(Boc)-OH, Fmoc-L-Tyr(tBu)-OH, Fmoc-L-Val-OH.
[0127] Synthesis of Fmoc-Ser(tBu)-King Resin:
[0128] Weigh 12.95 g of Wang resin (Tianjin Nankai Hecheng Technology Co., Ltd.) with a degree of substitution of 0.58 mmol / g, add it to a solid-phase reaction column, add 100 mL of DCM to swell the resin for 30 minutes, and was...
Embodiment 2
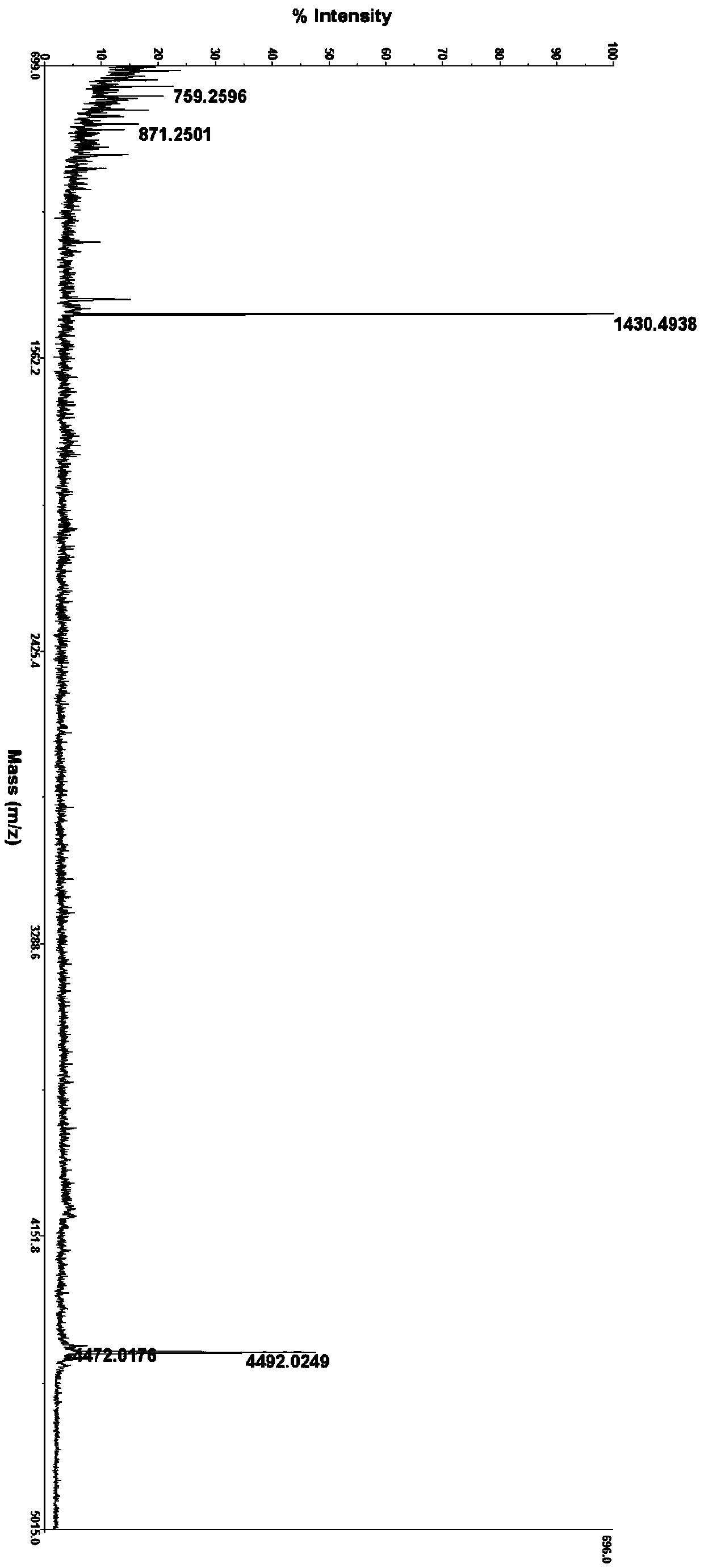
[0138] Preparation of glucagon derivative FC382K14W07:
[0139]
[0140] HSQGT FTSDY SKYXD SQAAQ DFVQW LMNGG PSSGA PPPS-OH
[0141] X14=K(octadecanoyl-γE) (SEQ ID NO.81)
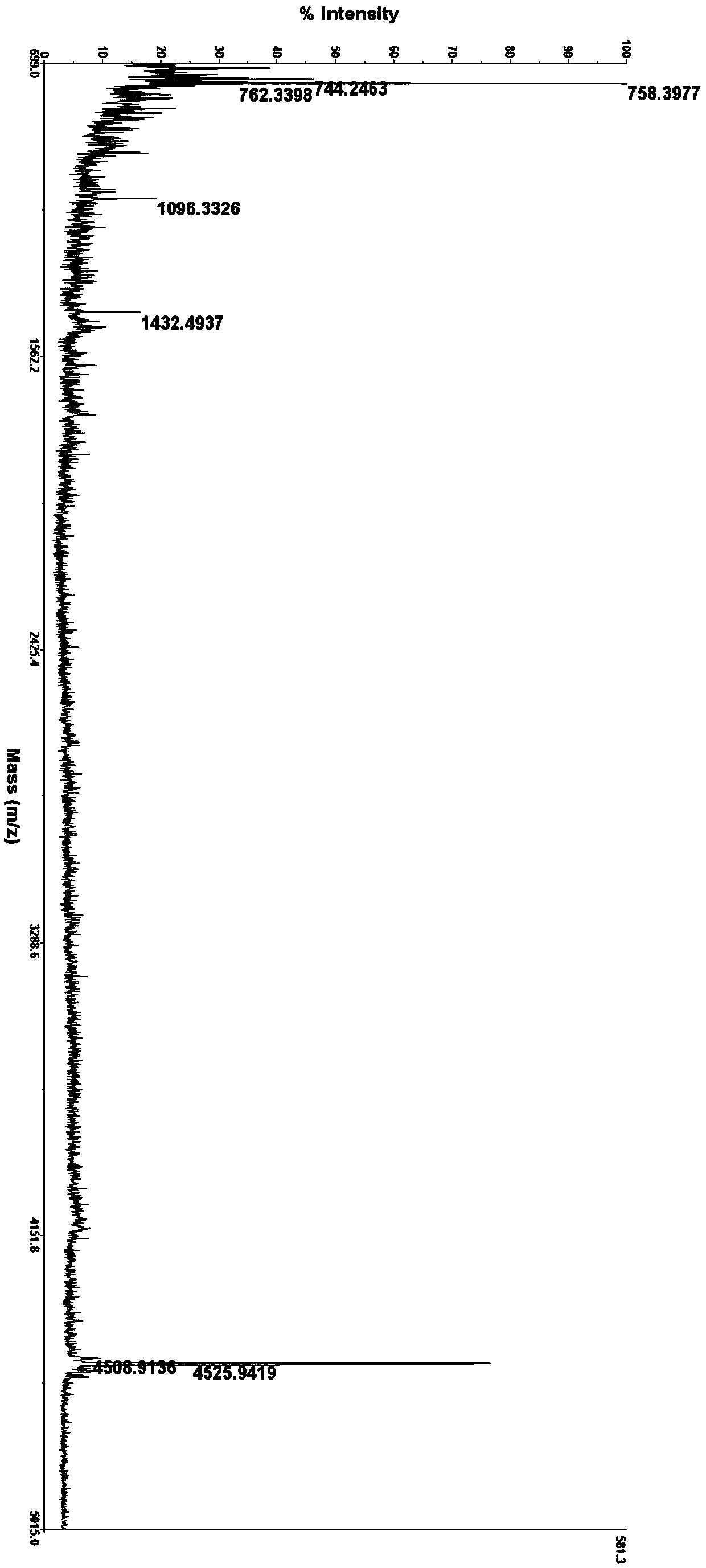
[0142] The synthesis method is the same as in Example 1, wherein K14 is coupled with Fmoc-Lys(Staroyl-Glu-OtBu)-OH (Hangzhou Hesu Chemical Technology Co., Ltd.), the obtained crude peptide is purified by RP-HPLC, and finally freeze-dried to obtain Refined peptide (97.3%). MS: m / z 4569.02 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com