FGF21 Fusion Proteins and Method of Inhibiting Degradation Thereof
A technology of FGF21 and fusion protein, which is applied in the direction of fusion polypeptide, chemical instruments and methods, animal/human protein, etc., can solve the problems of time-consuming and other problems, and achieve the effect of simple operation, inhibition effect and reduction of degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
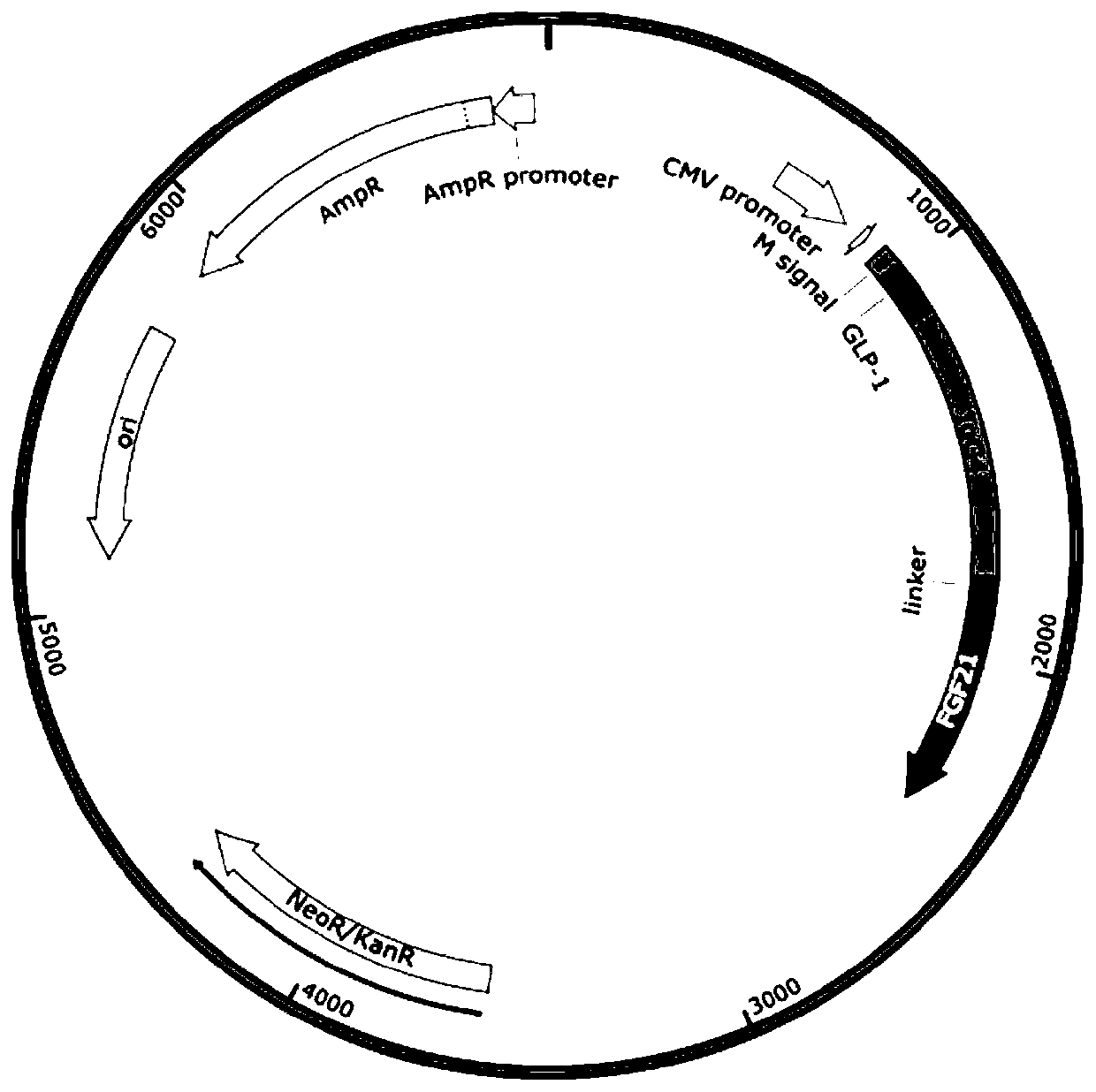
[0046] Embodiment 1: Construction of expression vector
[0047] A genetically engineered cell line that expresses GLP-1 analogues and FGF21 analogues is constructed.
[0048] According to CHO's preferred codon optimization fusion protein sequence A-B-C-D-E-F, where A is the signal peptide, B is the glucagon-like peptide-1 variant (GLP-1), C is the linker peptide, D is the IgG4 variant, and E is the linker Peptide, F is FGF21 variant.
[0049] A: signal peptide
[0050] Nucleotide sequence (SEQ ID NO: 1)
[0051] atgcggtttttcttcgtgttcctggccatcgtgctgtttcagggcatccacgga
[0052] Amino acid sequence (SEQ ID NO:2)
[0053] MRFFFVFLAIVLFQGIHG
[0054] B: GLP-1 variant
[0055] Nucleotide sequence (SEQ ID NO:3)
[0056] cacggagaaggaacctttacctccgacgtgtcttcttacctggaggaacaggcagctaaggagtttatcgcttggctggtgaaaggaggagga
[0057] Amino acid sequence (SEQ ID NO:4)
[0058] HGEGFTTSDVSSYLEEQAAKEFIAWLVKGGG
[0059] C: connecting peptide
[0060] Nucleotide sequence (SEQ ID NO:5)
[00...
Embodiment 2
[0076] Example 2: Construction of CHO stably transfected cell pool
[0077] A CHO stably transfected cell pool expressing fusion protein A-B-C-D-E-F was constructed.
[0078] The expression vector constructed in Example 1 was linearized with restriction endonuclease, precipitated with ethanol, extracted with phenol and chloroform, and dissolved for use.
[0079] After the CHOK1 host cells were revived and cultured with Cellvento CHO 200 medium, when the cell density was about 8×10 5 Cells / mL were harvested for transfection. Transfected cells about 1×10 7 Cell, about 40 μg of linearized plasmid, was transfected by electroporation (Bio-Rad, Gene pulser Xcell). Cells were cultured in 20 mL Cellvento CHO 200 medium after electric shock. On the second day of culture, the cells were collected by centrifugation, and resuspended in 20 mL Cellvento CHO 200 medium for pressurized culture. When the cell density is about 0.6×10 6 cell / mL, the obtained mixed clones were subcultured w...
Embodiment 3
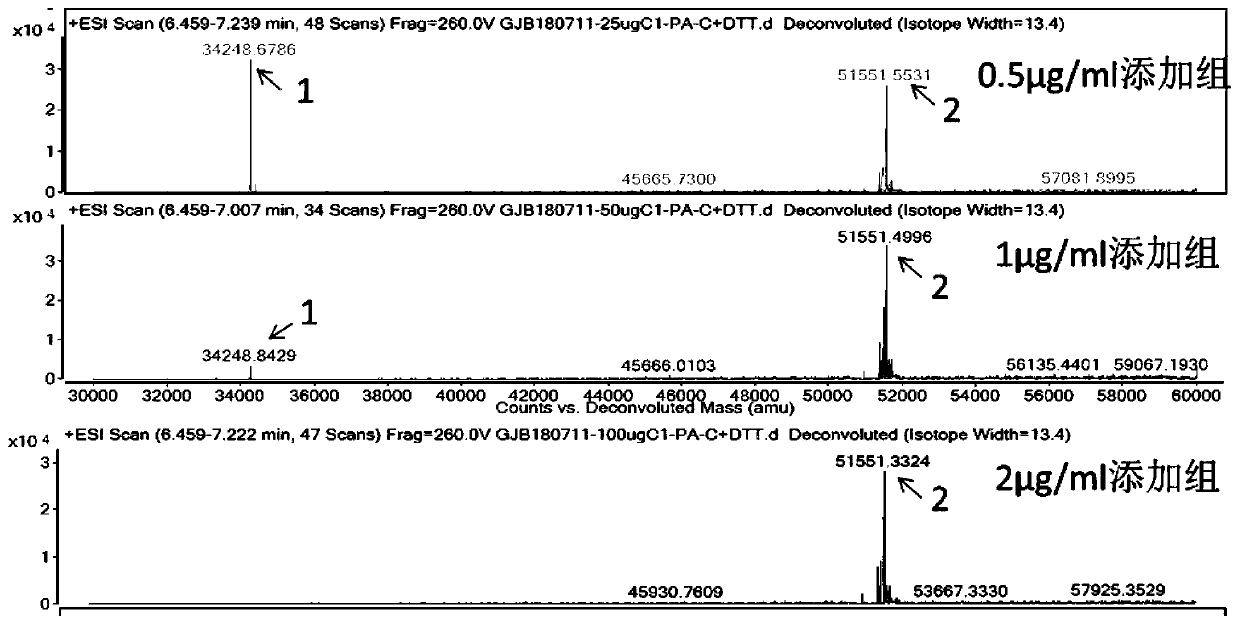
[0080] Embodiment 3: mass spectrometry detection
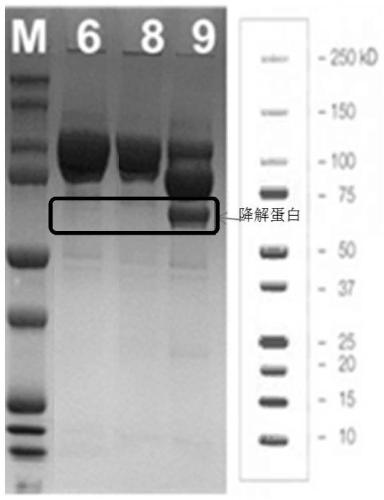
[0081] The obtained CHOK1 stably transfected cell pool was inoculated into Cellvento 200 medium, and C1 inhibitor was added to the medium, respectively high concentration addition group (2 μg / mL), medium concentration addition group (1 μg / mL), low concentration addition group ( 0.5μg / mL), 37°C, 8% CO 2 After culturing for 3 days under the same conditions, the temperature was lowered to 30° C. to continue culturing for 4 days. After protein A purification and DTT reduction, mass spectrometry detection was performed. The molecular weight of the intact undegraded protein is about 51.5Kda, and the molecular weight of the main degraded protein is about 34.2Kda.
[0082] The results showed that the high-concentration addition group could inhibit the degradation of FGF21 fusion protein, and the inhibitory effect was dose-dependent. The higher the addition concentration, the less the proportion of degraded protein (see figure 2 )....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com