Method for preparing human pancreatic glucagons like polypeptide - 1, and fusion proten of human serum albumin, and products
A human serum albumin and fusion protein technology, applied in the field of long-acting fusion protein drugs, can solve the problems of clinical application limitations and difficulty in wide application, and achieve the effect of avoiding the stability of fusion proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
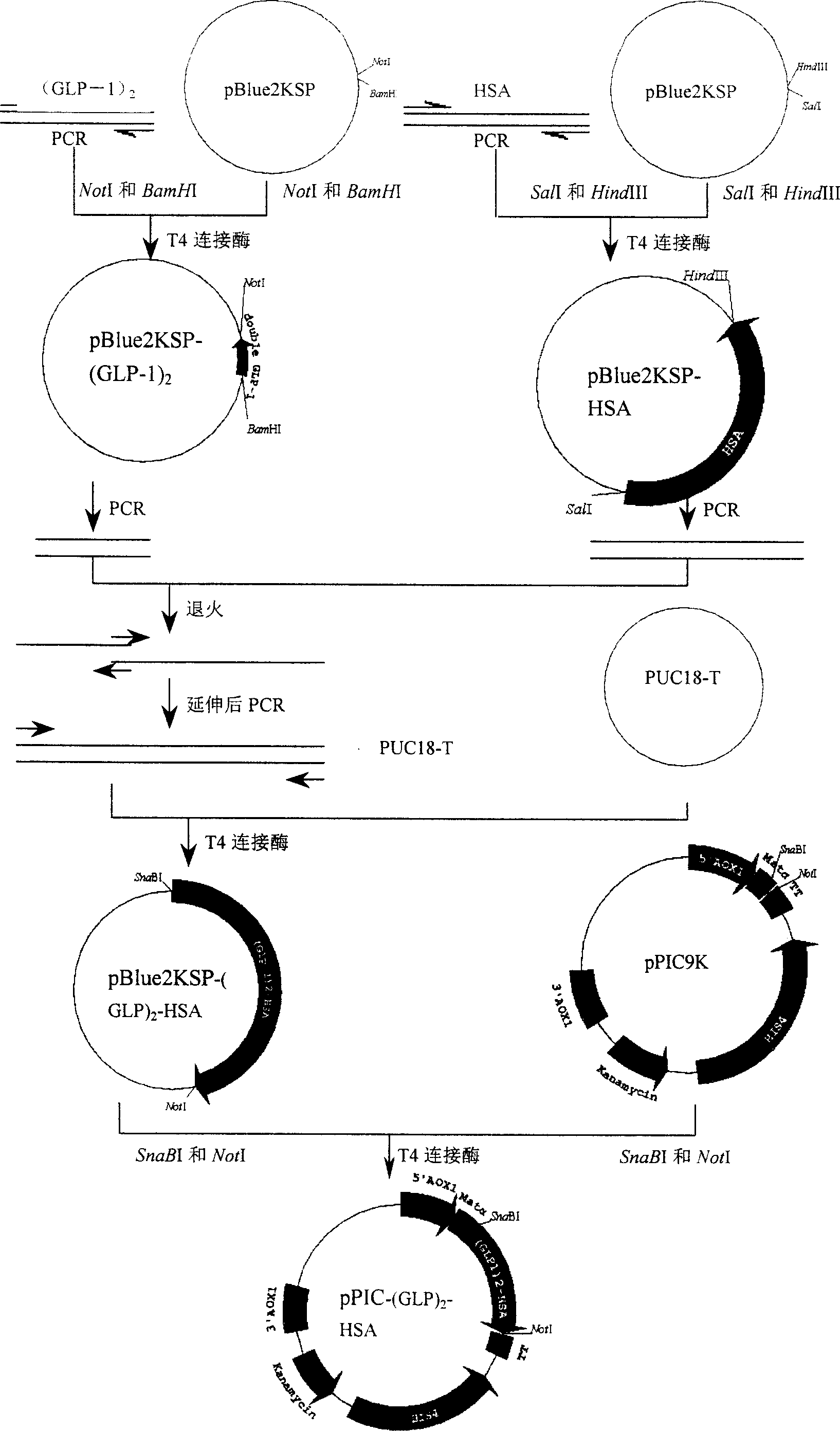
[0026] Example 1: (GLP-1) 2 cDNA cloning
[0027] Synthetic (GLP-1) 2cDNA (completed by Shanghai Sangon Bioengineering Technology Service Co., Ltd.). Artificially synthesized (GLP-1) 2 cDNA as template, amplified by PCR (GLP-1) 2 cDNA, the primers used are as follows:
[0028] PG1: 5'TGC GGATCC CACGGTGAAGGTACTTTCACTTCTGA-3'
[0029] PG2: 5'-ATA GCGGCCGC TTAACGACCCTTAACCAACCAAGC-3'
[0030] The PCR method is as follows: Add to the 50 μl reaction system: 1.5 μl of 10 μmol / L PG1 and PG2 primers, 5 μl of 2 mmol / L dNTP, 1 μl of 10×pfu Buffer, 0.5 μl of 5 U / μl pfu DNA polymerase (dNTP, 10×pfu Buffer and pfu DNA polymerase were purchased from Shanghai Sangon Bioengineering Technology Service Co., Ltd., the same below), (GLP-1) 2 cDNA 1μg, add double distilled water to make up 50μl, in PTC-100 of MJ Research TM On the PCR instrument (the same below), the PCR conditions are: denaturation at 95°C for 10 minutes, denaturation at 94°C for 1 minute, annealing at 60°C for 1 min...
Embodiment 2
[0032] Example 2: Cloning of HSA cDNA
[0033] HSA cDNA was amplified from the human fetal liver cDNA library by PCR, and the primers used were:
[0034] PH1: 5'-AG GTC GAC GATGCACACAAGAGTGAGGTTGCTC-3'
[0035] PH2: 5'-GC AAGCTT TTATAAGCCTAAGGCAGCTTGACTT-3'
[0036] The PCR method is as follows: Add to the 50 μl reaction system: 1.5 μl of 10 μmol / L PH1 and PH2 primers, 5 μl of 2 mmol / L dNTP, 1 μl of 10×pfu Buffer, 0.5 μl of 5 U / μl pfu DNA polymerase, human fetal Liver cDNA library 1 μg, add double distilled water to make up 50 μl; PCR conditions are: denaturation at 95°C for 5 minutes, denaturation at 94°C for 1 minute, annealing at 60°C for 1 minute, extension at 72°C for 90 seconds, cycle 30 times;
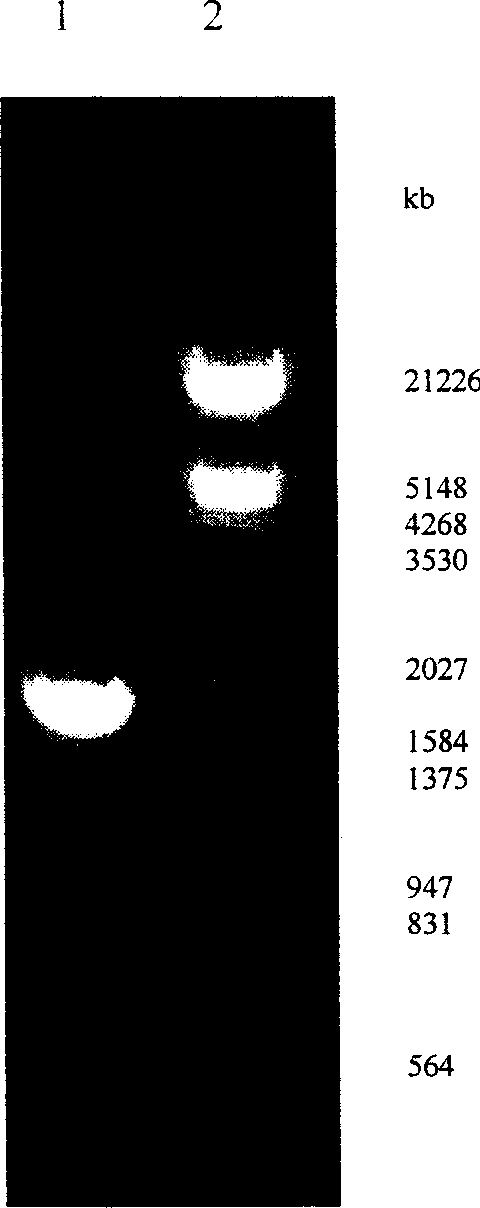
[0037] The reaction product was analyzed by agarose gel electrophoresis, and the target band appeared in the loading lane, and the 1.8kb target fragment was purified with a PCR fragment gel recovery kit, and the purified target fragment and the carrier pBlue2KSP were doubl...
Embodiment 3
[0038] Embodiment 3: (GLP-1) 2 Cloning of cDNA and HSA cDNA fusion gene
[0039] (GLP-1) 2 For PCR amplification of cDNA, the primers used are as follows:
[0040] PG3: 5’-GAGA GGTACC TAAAAAGACACGGTGAAGGTACTTTCACT-3'
[0041] PG4: 5'-ACCTCACTCTTGTGTGCATCCCTTCCTTTACTAACCATG-3'
[0042] The PCR method is as follows: 50 μl reaction system was added: 1.5 μl of 10 μmol / L Pf1 and Pf2 primers, 5 μl of 2 mmol / L dNTP, 1 μl of 10×pfu Buffer, 0.5 μl of 5 U / μl pfu DNA polymerase, pBlue2KSP- (GLP-1) 2 1ng, add double distilled water to make up 50μl; PCR conditions are: denaturation at 95°C for 5 minutes, annealing at 65°C for 1 minute, extension at 72°C for 90 seconds, denaturation at 94°C for 1 minute, cycle 30 times;
[0043] PCR amplification of HSA cDNA, the primers used are as follows:
[0044] PH3: 5'-CATGGTTAGTAAAAGGAAGGGATGCACACAAGAGTGAGGT-3'
[0045] PH4: 5'-TGAA CCGCGGCCGC TTATAAGCCTAAGGCAGCTTG-3'
[0046] The PCR method is as follows: Add to the 50 μl reaction system...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com