Application of butyric acid producing beneficial bacterium in preparing preparation for preventing and treating severe disease gut barrier injury and post-injury complication
The technology of intestinal barrier and beneficial bacteria is applied in the application field of butyric acid-producing beneficial bacteria in the preparation of preparations for preventing and treating intestinal barrier damage and complications after damage in severe diseases, and can solve the problem that the proximal intestinal tract cannot be effectively achieved, and the unpleasant odor , poor dose control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0079] Preparation of Medicine Preparation Example 1 Oral Fungi Live Bacteria Powder
[0080] 1 Preparation of bacteria powder and identification of strains
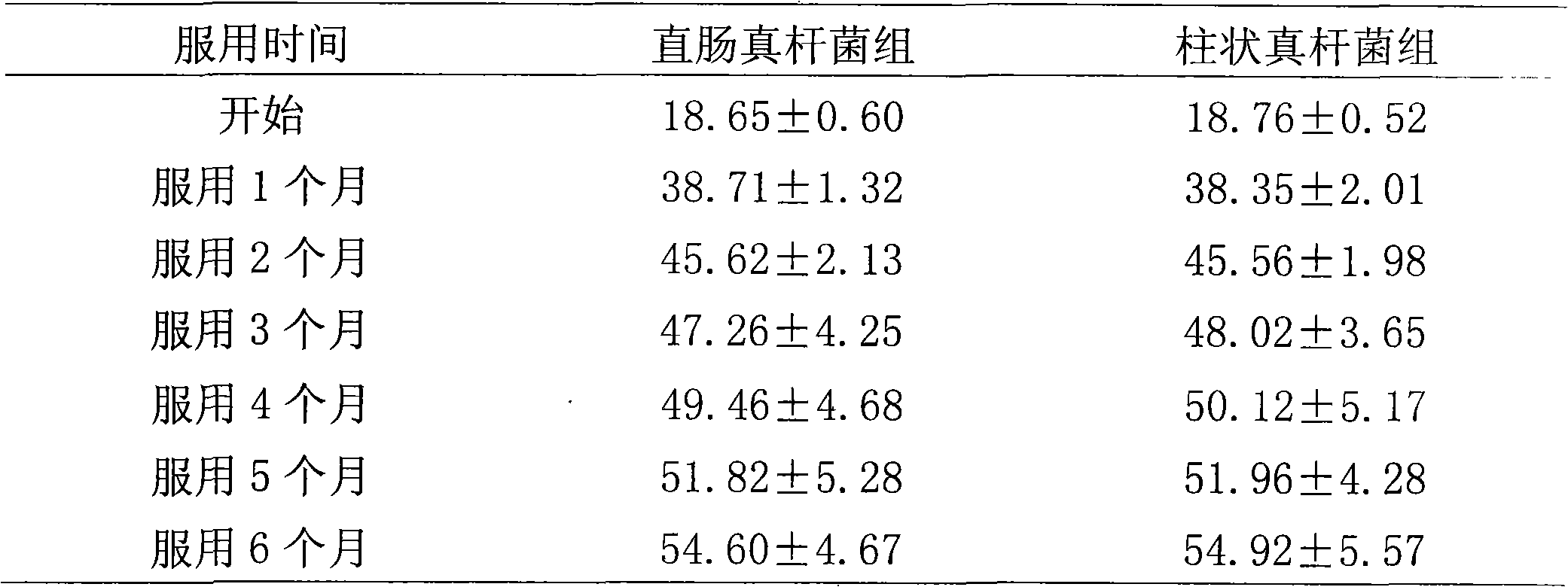
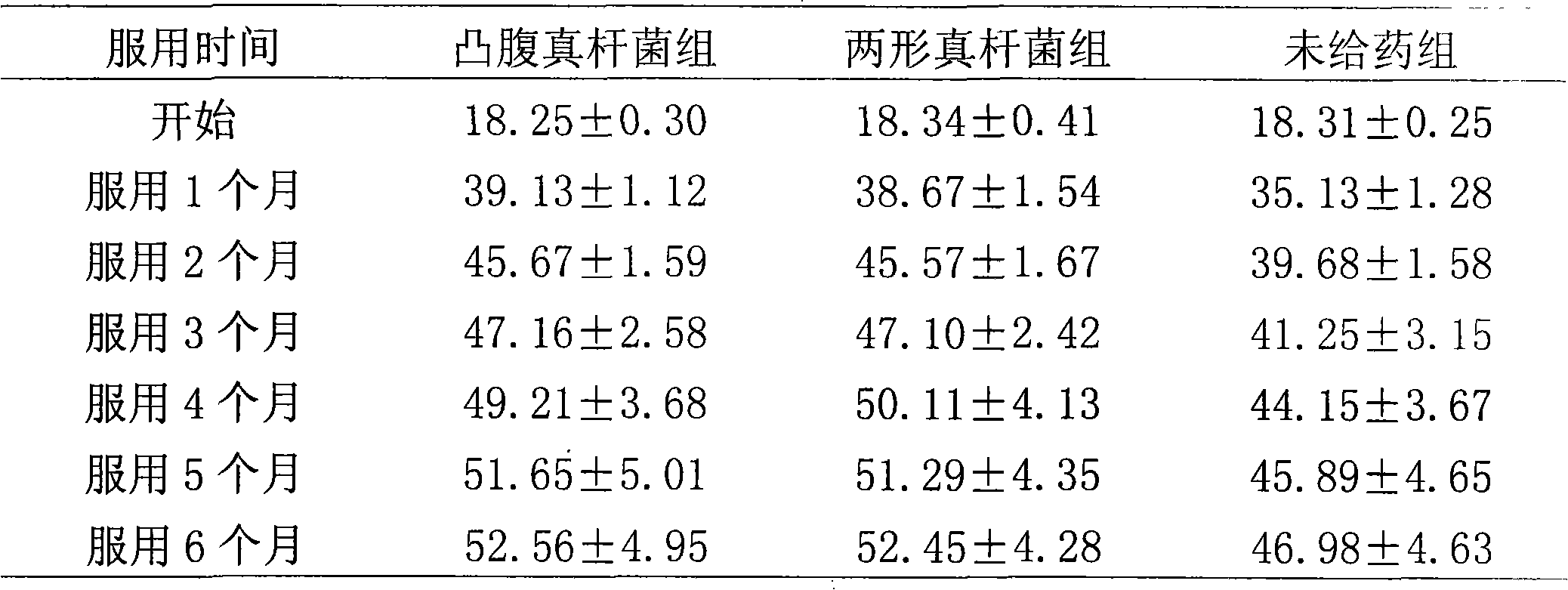
[0081] Take healthy baby feces, then put the feces in a sterilized anaerobic bottle, blow in nitrogen gas and mix well at the same time, quickly take 2 grams of feces from it and add it to 18mL sterilized diluent, blow in nitrogen gas and mix well at the same time. In the console, do 10 -1 、10 -2 、10 -3 、10 -4 , 10 -5 , 10 -6 , 10 -7 Gradient dilution, take 10 -5 , 10 -6 , 10 -7 Three dilution gradients, spread on the Eubacterium selective single colony isolation solid medium, place in an anaerobic tank, anaerobic culture at 37°C for 48 hours, select four single colonies with good growth and inoculate them into the Eubacterium liquid The expansion medium was placed in an anaerobic tank, and the expansion culture was carried out at 37° C. for 48 hours in anaerobic condition. After the obtained culture solution ...
preparation Embodiment 2
[0098] Preparation of oral Clostridium tertiary live bacteria powder and oral Clostridium butyricum (Clostridium butyricum) live bacteria powder 1 preparation of bacteria powder and identification of strains
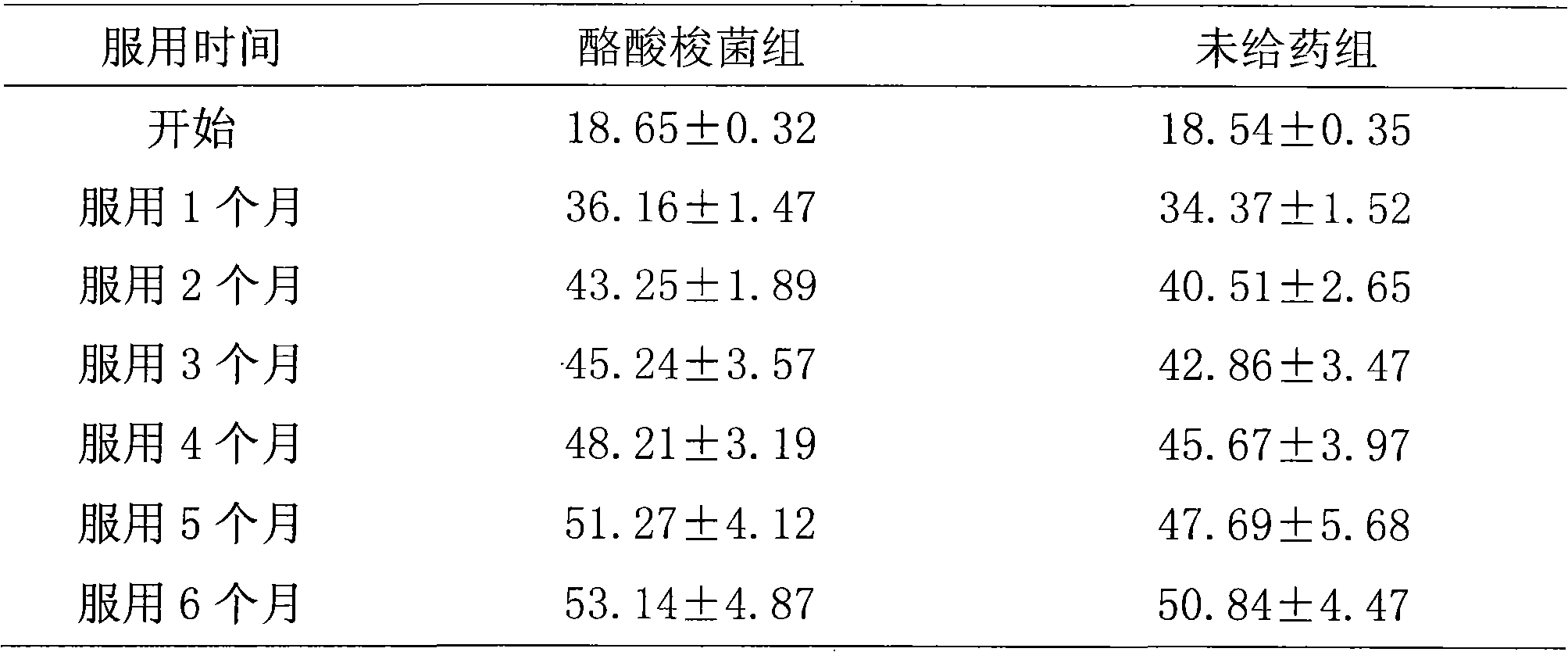
[0099] Take healthy baby feces, then put the feces in a sterilized anaerobic bottle, blow in nitrogen gas and mix well at the same time, quickly take 2 grams of feces from it and add it to 18mL sterilized diluent, blow in nitrogen gas and mix well at the same time. In the console, do 10 -1 、10 -2 、10 -3 、10 -4 , 10 -5 , 10 -6 , 10 -7 Gradient dilution, take 10 -5 , 10 -6 , 10 -7 Three dilution gradients, 80°C water bath for 13 minutes, spread on the solid medium for Clostridium selective single colony isolation, place in an anaerobic tank, culture at 37°C for 48 hours, and select three single colonies with good growth They were respectively inoculated into Clostridium liquid expansion medium, placed in an anaerobic tank, and amplified at 37° C. for 48 hours unde...
preparation Embodiment 3
[0115] Medicine Preparation Example 3 Preparation of Bifidobacterium and Lactobacillus Powder and Fermentation Supernatant
[0116] In order to further illustrate the novelty and creativity of the present invention, Bifidobacterium and Lactobacillus are used for comparative research in the application effect examples. The selected bifidobacterium is Bifidobacterium infantis DF96102 with preservation number CGMCC0313.2, and the lactobacillus is Lactobacillus acidophilus DF96106 with preservation number CGMCC0313.4.
[0117] 1 Preparation of Bifidobacteria powder and fermentation supernatant
[0118] Culture medium is (medium is not limited to that described in the present invention, known culture medium all can): peptone 15.0g, yeast powder 2.0g, glucose 20.0g, soluble starch 0.5g, sodium chloride 5.0g, 5% cysteine Amino acid 10.0mL, tomato extract 400.0mL, Tween 80 1.0mL, liver extract 80.0mL, agar 20.0g, distilled water 520.0mL, adjust pH to 7.0, sterilize at 115°C for 20min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com