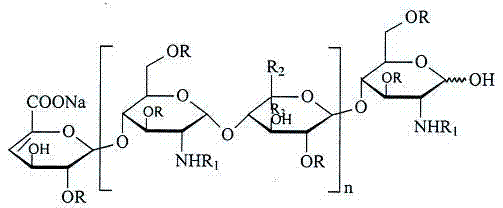
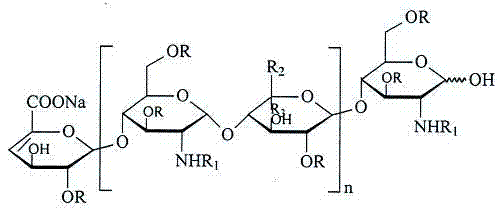
Enoxaparin sodium compound and preparation method thereof
A technology of enoxaparin sodium and enoxaparin, which is applied in the field of enoxaparin sodium and its preparation, can solve the problems of waste water pollution, affecting the esterification rate, and low yield, and achieve high yield, short reaction time, and easy operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Take 10g of heparin sodium, 50mg of sodium chloride, and 25g of benzethonium chloride, mix them evenly, place them in a microwave reactor with a power of 150W, and react at a temperature of 60°C for 30min, then cool to obtain 30g of heparin quaternary ammonium salt;
[0038] Using the method of US Patent No. 5,389,618, the quaternary ammonium salt of heparin was esterified, cracked, and purified to obtain 18.5 g of enoxaparin.
[0039]
[0040] The product tests are as follows:
[0041] Source of reference substance: Imported enoxaparin sodium injection (Kesai, batch number: 110601-J), extracted and refined by Suzhou Erye Pharmaceutical Co., Ltd. according to enoxaparin sodium raw material refining method.
[0042] 1. High resolution mass spectrometry
[0043] Mass spectrometry system: Shimadzu LCMS-IT-TOF
[0044] Off-line positive ion mode, spray voltage 2.0kV, spray gas flow rate 0.5L / min, CDL temperature 200°C, heating module temperature 200°C, detector volta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com