Enoxaparin sodium injection and preparation process thereof
A technology for enoxaparin sodium and injection, which is applied in the field of enoxaparin sodium injection and its preparation, can solve problems such as the stability of enoxaparin sodium injection that cannot be well solved, and achieves improved stability and improved stability. Good, simple and applicable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
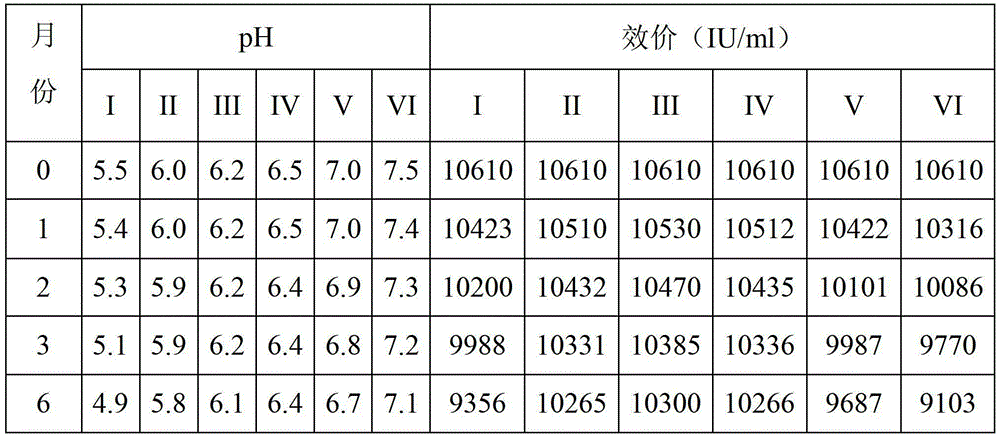
Image
Examples
Embodiment 1
[0029] Prepare enoxaparin sodium injection according to the following formula:
[0030]
[0031] According to the above formula, first dissolve L-cysteine in water for injection cooled to room temperature to obtain a solution of L-cysteine; quickly dissolve enoxaparin sodium and vitamin C in the above-mentioned L-cysteine In the solution, use 0.5mol / L NaOH to adjust the pH of the solution to 6.2, then filter it through a sterile filtration system, and aseptically fill it to obtain 5000 0.6ml / enoxaparin sodium injection injections .
Embodiment 2
[0033] Prepare enoxaparin sodium injection according to the following formula:
[0034]
[0035] According to the above formula, first dissolve L-cysteine in water for injection cooled to room temperature to obtain a solution of L-cysteine; quickly dissolve enoxaparin sodium and vitamin C in the above-mentioned L-cysteine In the solution, use 0.5mol / L NaOH to adjust the pH of the solution to 6.2, then filter it through a sterile filtration system, and aseptically fill it to obtain 5000 0.6ml / enoxaparin sodium injection injections .
Embodiment 3
[0037] Prepare enoxaparin sodium injection according to the following formula:
[0038]
[0039] According to the above formula, first dissolve L-cysteine in water for injection cooled to room temperature to obtain a solution of L-cysteine; quickly dissolve enoxaparin sodium and vitamin C in the above-mentioned L-cysteine In the solution, use 0.5mol / L NaOH to adjust the pH of the solution to 6.2, then filter it through a sterile filtration system, and aseptically fill it to obtain 5000 0.6ml / enoxaparin sodium injection injections .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com