Method for directly producing enoxaparin sodium from crude product heparin sodium
A technology of enoxaparin sodium and heparin sodium, which is applied in the field of preparation of enoxaparin sodium, can solve the problems of unstrict control of starting materials, high sulfate content in finished products, and large amount of organic solvents used, so as to achieve stable product quality Controllable, stable product quality, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
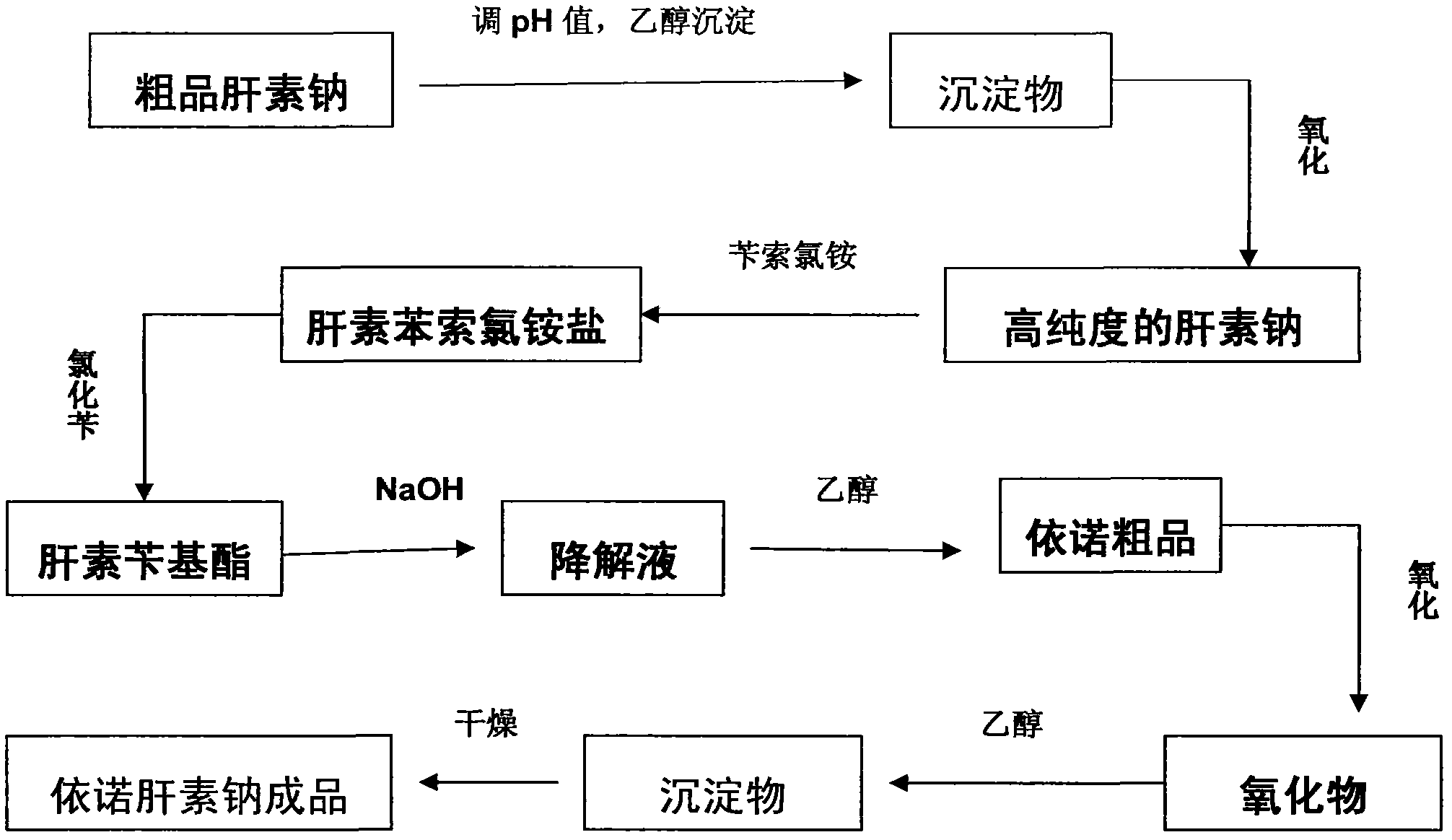
[0029] Such as figure 1 As shown, the process described in this embodiment includes the following steps:
[0030] Take 100Kg of crude product heparin sodium, add purified water to dissolve it into a crude product sodium heparin aqueous solution with a concentration of 10wt%; use hydrochloric acid or sodium hydroxide with a concentration of 4mol / L to adjust the pH value of the solution to 5.2, and then add 0.4 times the volume of the solution for medicinal Ethanol, adjust the temperature to 0.5°C, let it stand for 6 hours, collect the precipitate a, add 5 times the volume of the precipitate a purified water, after it is completely dissolved, add 0.2v% of the solution volume of hydrogen peroxide to oxidize for 6 hours, filter with a filter membrane , add 2 times the volume of ethanol to precipitate the solution, place the precipitate for 6 hours to obtain precipitate b; add purified water to dissolve the precipitate b to form a heparin sodium solution with a concentration of 10 ...
Embodiment 2
[0033] Enoxaparin sodium is realized by the following process. Take 100Kg of crude heparin sodium, add purified water to dissolve it into a crude product heparin sodium aqueous solution with a concentration of 15wt%, use hydrochloric acid or sodium hydroxide with a concentration of 4mol / L to adjust the pH value of the solution to 6.7, Then add medicinal ethanol 0.8 times the volume of the solution, adjust the temperature to 9.5°C, let it stand for 24 hours, collect the precipitate a, add 15 times the volume of purified water of the precipitate a, after completely dissolving, add 1v% hydrogen peroxide of the solution volume Oxidize for 12 hours, filter with a filter membrane, add ethanol in an amount twice the volume of the solution to precipitate, and place the precipitate for 12 hours to obtain precipitate b; add purified water to precipitate b to dissolve it into a heparin sodium solution with a concentration of 15 wt%, and take the precipitate Benzethonium chloride with 4 ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com