Gliclazide sustained release tablet
A technology of gliclazide and sustained-release tablets, applied in the field of sustained-release tablets for the treatment of diabetes, can solve the problems of complicated production process of osmotic pump controlled-release tablets, unfavorable for large-scale production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
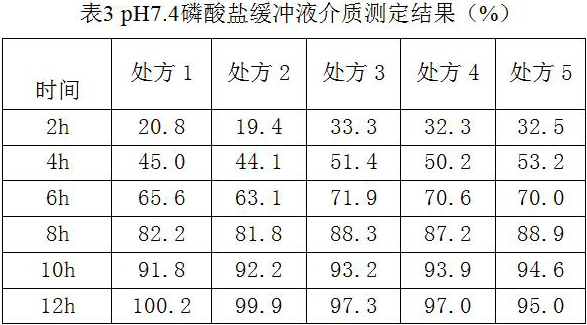
[0036]
[0037] Preparation:
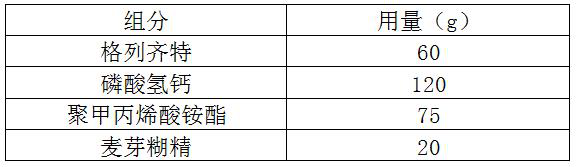
[0038] a. Mix gliclazide, calcium hydrogen phosphate and maltodextrin evenly to obtain a mixture;
[0039] b. Wet granulate the above mixture, and granulate to obtain drug granules;
[0040] c. Mix the above drug granules with polyammonium methacrylate evenly, and press into tablets.
Embodiment 2
[0042]
[0043] Preparation:
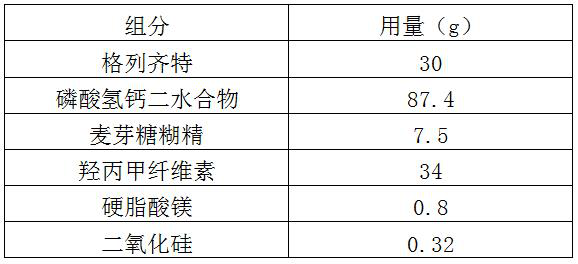
[0044] a. Mix gliclazide, calcium hydrogen phosphate and maltodextrin evenly to obtain a mixture;
[0045] b. Wet granulate the above mixture, and granulate to obtain drug granules;
[0046] c. Mix the above drug granules with polyammonium methacrylate evenly, and press into tablets.
Embodiment 3
[0048]
[0049] Preparation:
[0050] a. Gliclazide, calcium hydrogen phosphate, calcium sulfate and sorbitol are mixed uniformly to obtain a mixture;
[0051] b. Wet granulate the above mixture, and granulate to obtain drug granules;
[0052] c. Mix the above drug granules with polyammonium methacrylate evenly, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com