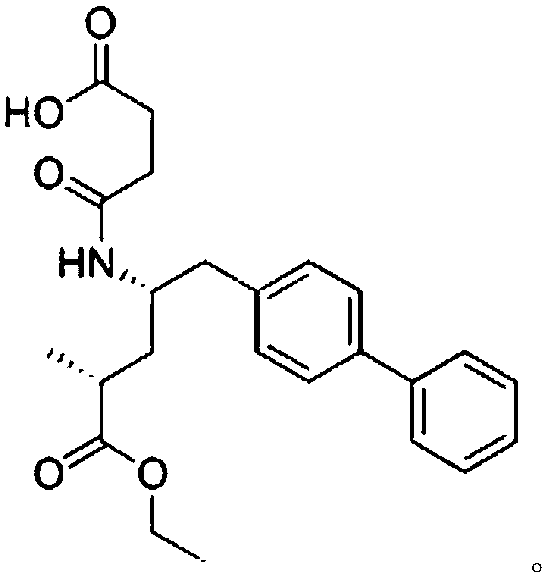
Chromatographic analysis method for AHU377 and AHU377 isomers
A technology of AHU377, 1.AHU377, applied in the field of drug analysis, can solve the problems that have not yet been achieved, and achieve the effects of improving drug quality, controlling impurity content, and reducing potential risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
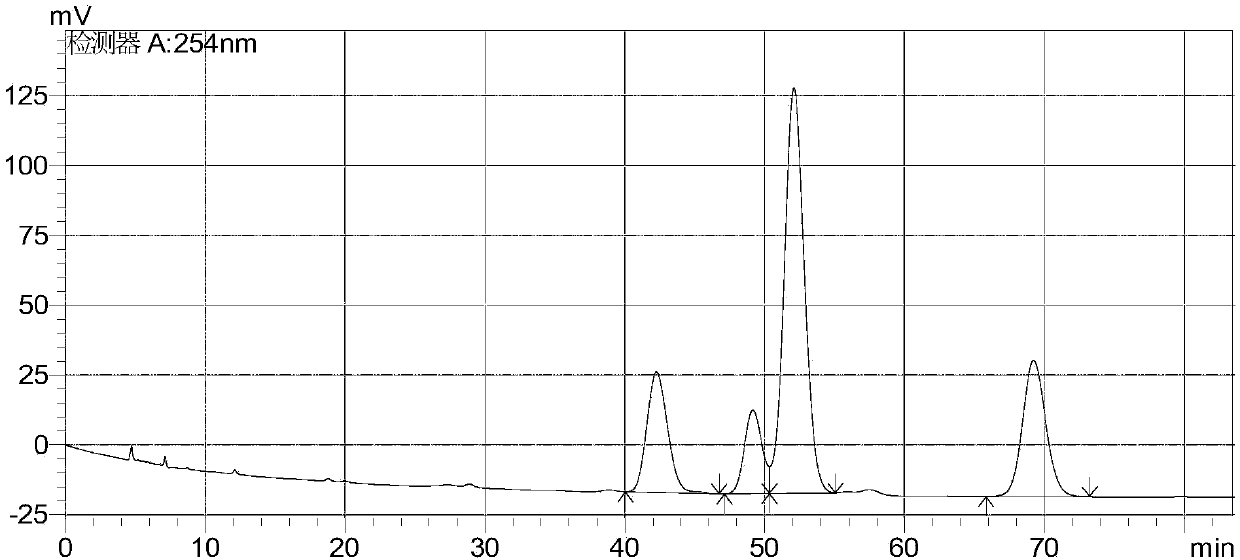
[0030] Testing conditions:
[0031] Instrument: Shimadzu 20A, Shimadzu 20AvT UV detector, detection wavelength: 254nm
[0032] Column: IC (3.5μm, 4.6×250mm) chiral column
[0033] Mobile phase: n-hexane-isopropanol-trifluoroacetic acid (90:10:0.1)
[0034] Diluent: n-hexane-isopropanol-trifluoroacetic acid (90:10:0.1)
[0035] Flow rate: 0.8mL / min
[0036] Column temperature: 40℃
[0037] Injection volume: 10μL
[0038] Detection steps:
[0039] Take 12.5mg each of AHU377 and AHU377-C mixture, AHU377-A and AHU377-B mixture, accurately weigh them, place them in a 50mL measuring flask, add mobile phase to dissolve and dilute to the mark, shake well, as a mixed stock solution. Take another 25 mg of the sample to be tested, accurately weigh it, and place it in a 50 mL measuring bottle. After adding an appropriate amount of mobile phase to dissolve, add 0.5 ml of the above-mentioned mixed stock solution, add mobile phase to dilute to the mark, and shake it as a system suitability solution.
[0...
Embodiment 2
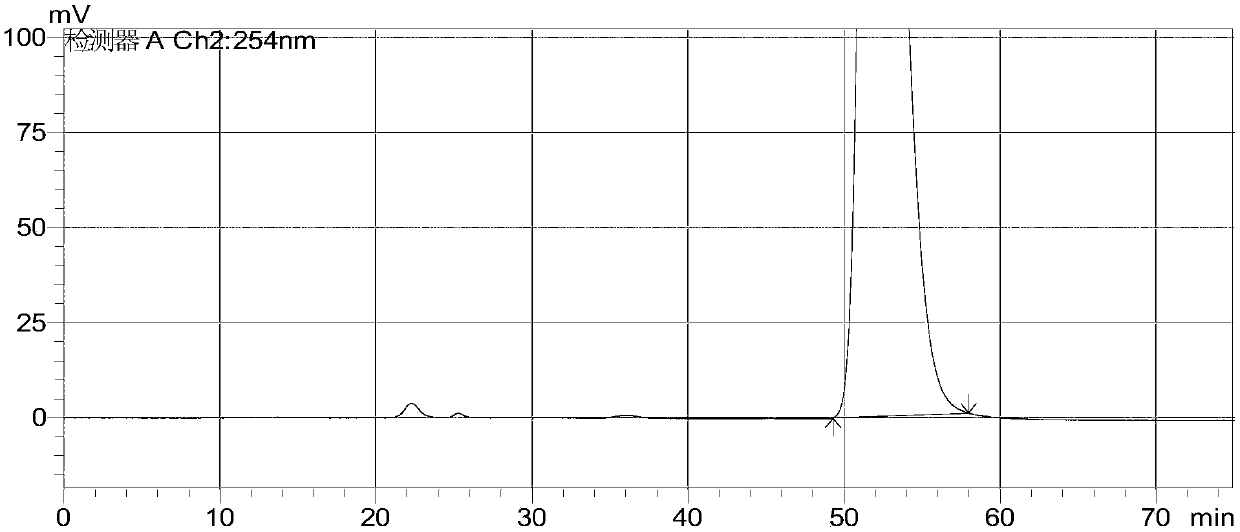
[0042] Testing conditions:
[0043] Instrument: Shimadzu 20A, Shimadzu 20AvT UV detector, detection wavelength: 254nm
[0044] Column: IC (3.5μm, 4.6×250mm) chiral column
[0045] Mobile phase: n-hexane-isopropanol-trifluoroacetic acid (90:10:0.1)
[0046] Diluent: n-hexane-isopropanol-trifluoroacetic acid (90:10:0.1)
[0047] Flow rate: 0.8ml / min
[0048] Column temperature: 40℃
[0049] Injection volume: 10μL
[0050] Detection steps:
[0051] Take about 25mg of this product, accurately weigh it, put it in a 25ml measuring flask, add mobile phase to dissolve and dilute to the mark, shake it well, and use it as the test solution;
[0052] Precisely take 20μL of the test solution, and perform the detection and analysis according to the above detection conditions, see figure 2 . figure 2 The isomers AHU377-A, AHU377-B and AHU377-C were not detected in the test product. The optical purity of this product meets the quality requirements, and this method can be used for quality monitoring o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com