Method for separating and determining glipizide and impurities thereof by liquid chromatography
A technology for glipizide and its determination method, which is applied in the field of separation and determination of glipizide and its impurities by high-performance liquid chromatography, and can solve the problems of poor peak shape of impurities, failure to achieve baseline separation, and influence on integral calculation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
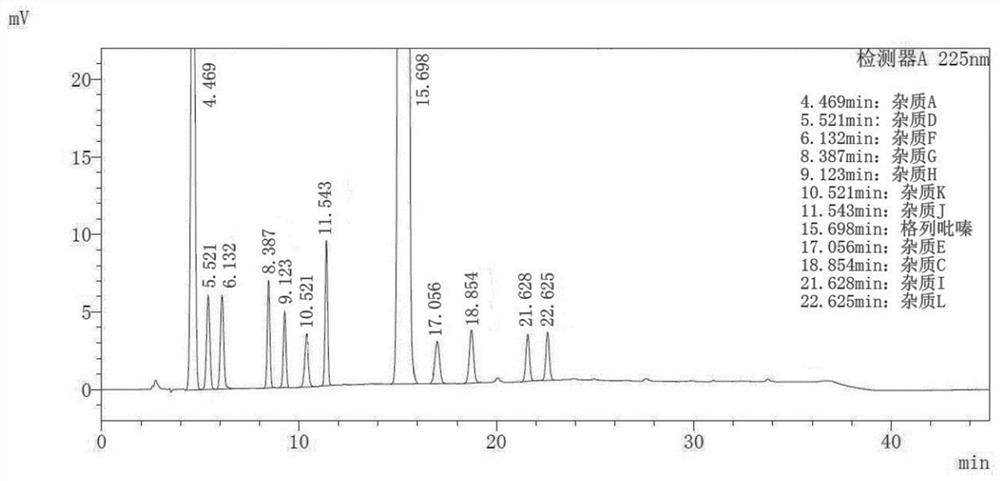
Embodiment 1
[0078] Instrument: Agilent 1260 InfinityII;
[0079] Stationary phase: Agilent ZORBAX SB-C18 (4.6mm×150mm, 5μm);
[0080] mobile phase:
[0081] Phase A: 0.015mol / L potassium dihydrogen phosphate, adjust the pH value to 3.7 with phosphoric acid;
[0082] Phase B: acetonitrile;
[0083] Phase C: Methanol;
[0084] Column temperature: 25°C;
[0085] Injection volume: 20μL;
[0086] Flow rate: 1.0mL / min;
[0087] Detection wavelength: 225nm;
[0088] Preparation of mobile phase:
[0089] Phase A: Take 2.04g of potassium dihydrogen phosphate, add water to dissolve and dilute to 1000mL, adjust the pH value to 3.7 with phosphoric acid;
[0090] Phase B: acetonitrile;
[0091] Phase C: Methanol.
[0092] Mobile phase gradient settings:
[0093] stage V A相 (%)
V B相 (%)
V C相 (%)
Elution time 0 65 25 10 7min 1 50 30 20 5min 2 40 35 25 6min 3 30 45 25 6min
[0094] Solution preparation:
[0095] Diluent: 0.015mol / L ...
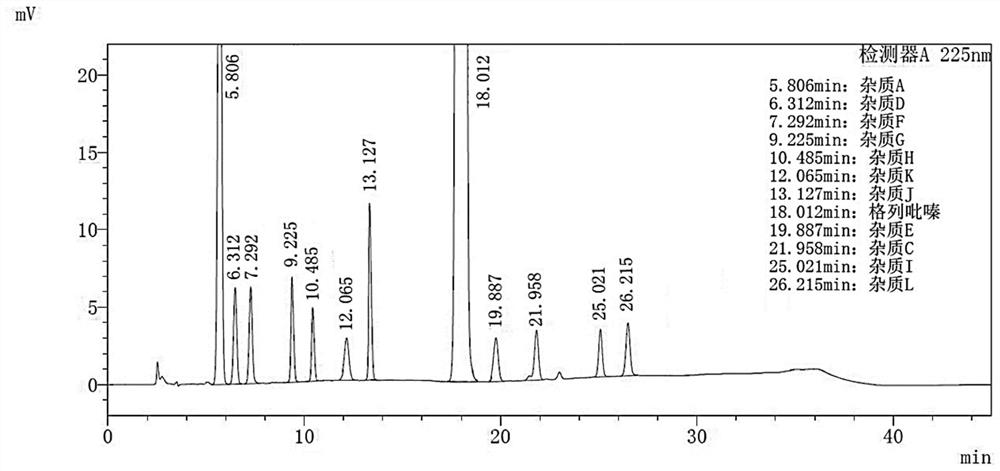
Embodiment 2
[0101] Instrument: Agilent 1260 InfinityII;
[0102] Stationary phase: Agilent ZORBAX SB-C18 (4.6mm×150mm, 5μm);
[0103] mobile phase:
[0104] Phase A: 0.010mol / L potassium dihydrogen phosphate, adjust the pH value to 3.5 with phosphoric acid;
[0105] Phase B: acetonitrile;
[0106] Phase C: Methanol;
[0107] Column temperature: 20°C;
[0108] Injection volume: 15μL;
[0109] Flow rate: 0.8mL / min;
[0110] Detection wavelength: 225nm;
[0111] Preparation of mobile phase:
[0112] Phase A: Take 1.36g of potassium dihydrogen phosphate, add water to dissolve and dilute to 1000mL, adjust the pH value to 3.5 with phosphoric acid;
[0113] Phase B: acetonitrile;
[0114] Phase C: Methanol.
[0115] Mobile phase gradient settings:
[0116] stage V A相 (%)
[0117] Solution preparation method and assay method are carried out with reference to embodiment 1, record chromatogram figure 2 . The chromatographic conditions of this embodiment can effectively disti...
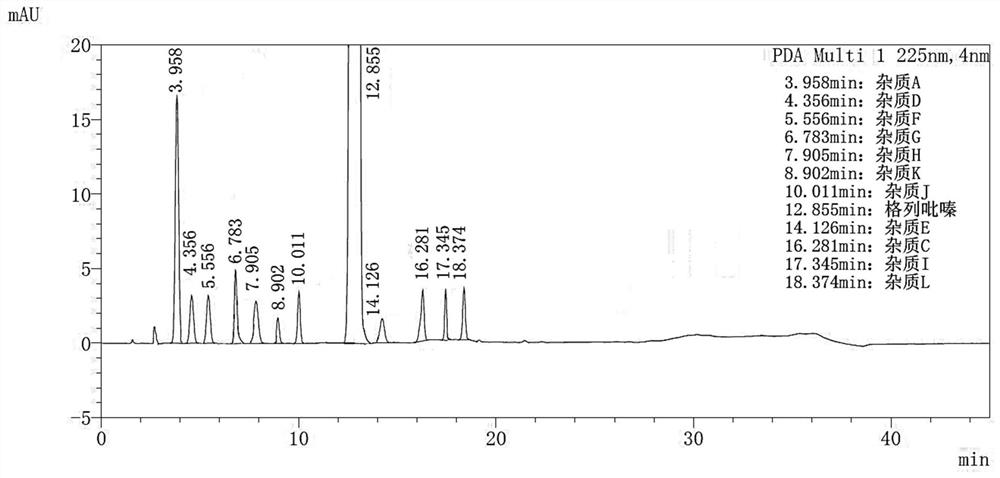
Embodiment 3
[0119] Instrument: Agilent 1260 Infinity II;
[0120] Stationary phase: Agilent ZORBAX SB-C18 (4.6mm×150mm, 5μm);
[0121] mobile phase:
[0122] Phase A: 0.018mol / L potassium dihydrogen phosphate, adjust the pH value to 4.0 with phosphoric acid;
[0123] Phase B: acetonitrile;
[0124] Phase C: Methanol;
[0125] Column temperature: 30°C;
[0126] Injection volume: 30μL;
[0127] Flow rate: 1.2mL / min;
[0128] Detection wavelength: 225nm;
[0129] Preparation of mobile phase:
[0130] Phase A: Take 2.45g of potassium dihydrogen phosphate, add water to dissolve and dilute to 1000mL, add phosphoric acid to adjust the pH to 4.0;
[0131] Phase B: acetonitrile;
[0132] Phase C: Methanol.
[0133] Mobile phase gradient settings:
[0134] stage V A相 (%)
[0135] Solution preparation method and assay method are carried out with reference to embodiment 1, record chromatogram image 3 . The chromatographic conditions of this embodiment can effectively distingui...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com