Method for producing enoxaparin sodium by using crude sodium heparin products
A technology for enoxaparin sodium and heparin sodium, which is applied in the field of biochemical raw material drug production, can solve problems such as unfavorable obtaining of high-quality final products, cumbersome steps, etc., and achieve the effects of low energy consumption, concise preparation process and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
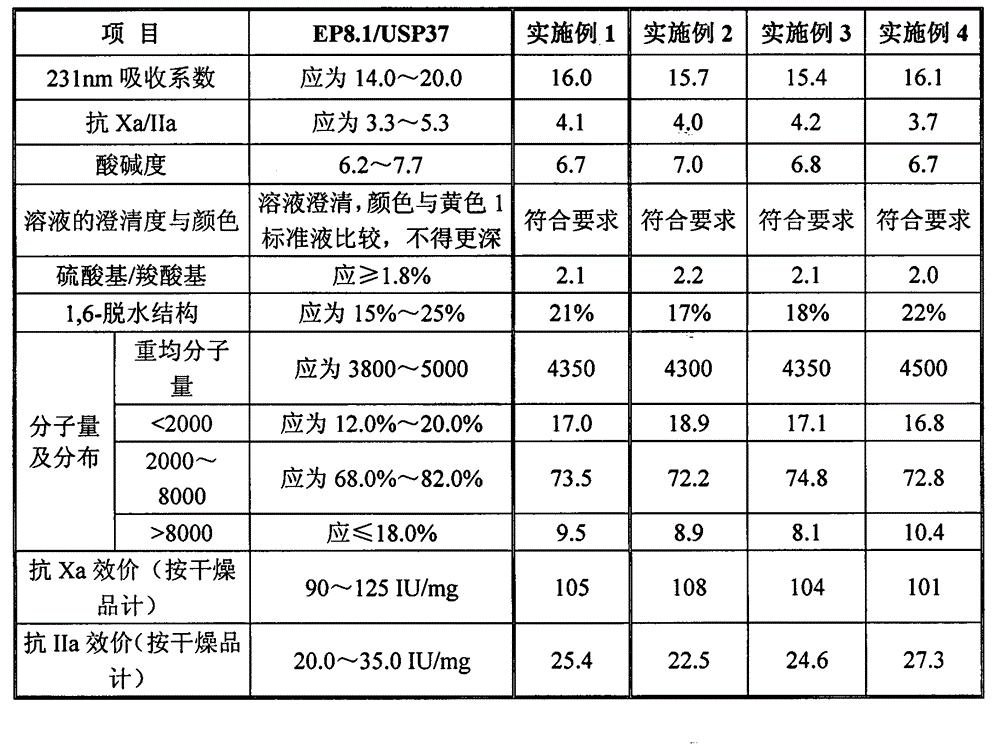
[0034] Take 1kg of crude heparin sodium, dissolve it in 8L of purified water, add 80g of sodium chloride, raise the temperature to 50°C, and at the same time adjust the pH value to 8.5 with 1mol / L sodium hydroxide solution, and perform salt hydrolysis for 2 hours under this condition. The salt solution was centrifuged to obtain heparin sodium pretreatment solution. Lower the temperature of the heparin sodium pretreatment solution to 28°C, add hydrogen peroxide, the volume of which is 1% of the heparin sodium pretreatment solution, stir evenly, add to a resin column equipped with 15L OC1074 resin for adsorption, and control the adsorption rate to 0.05BV / h, the resin after adsorption is first washed with 5.5% sodium chloride solution, the flow rate is 0.5BV / h, and washed to absorbance A 260nm ≤0.20,A 280nmWhen ≤0.20, use 12% sodium chloride solution to elute, the elution rate is 0.05BV / h, detect the refraction of the column liquid, start collecting the eluate when the refracti...
Embodiment 2
[0036] Take 1kg of crude heparin sodium, dissolve it in 10L of purified water, add 200g of sodium chloride, raise the temperature to 60°C, and adjust the pH value to 9.0 with 3mol / L sodium hydroxide solution, and perform salt solution for 3 hours under this condition , and the salt solution was centrifuged to obtain a heparin sodium pretreatment solution. Lower the temperature of the heparin sodium pretreatment solution to 32°C, add hydrogen peroxide, the volume of which is 2% of the heparin sodium pretreatment solution, stir evenly, add to a resin column equipped with 15L FPA98 resin for adsorption, and control the adsorption rate to 0.06BV / h, the resin after adsorption is first washed with 6% sodium chloride solution, the flow rate is 0.5BV / h, and washed to absorbance A 260nm ≤0.20,A 280nm When ≤0.20, elute with 12% sodium chloride solution, the elution rate is 0.06BV / h, detect the refraction of the column liquid, and start collecting the eluate when the refraction is grea...
Embodiment 3
[0038] Take 1kg of crude heparin sodium, dissolve it in 10L of purified water, add 200g of sodium chloride, raise the temperature to 50°C, and at the same time adjust the pH value to 8.5 with 6mol / L sodium hydroxide solution, and perform salt hydrolysis for 2 hours under this condition. The salt solution was centrifuged to obtain heparin sodium pretreatment solution. Lower the temperature of the heparin sodium pretreatment solution to 30°C, add hydrogen peroxide, the volume of which is 1.5% of the heparin sodium pretreatment solution, stir evenly, add to a resin column equipped with 15L FPA98 resin for adsorption, and control the adsorption rate to 0.05BV / h, the resin after adsorption is first washed with 5.5% sodium chloride solution, the flow rate is 0.5BV / h, and washed to absorbance A 260nm ≤0.20,A 280nm When ≤0.20, use 12% sodium chloride solution to elute, the elution rate is 0.05BV / h, detect the refraction of the column liquid, start collecting the eluate when the refr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com