Islamic enoxaparin sodium and method for producing and purifying same
A technology of enoxaparin sodium and heparin sodium, which is applied in the field of halal enoxaparin sodium and its production and purification, can solve the problems of thrombosis mortality and other problems, achieve the effects of improving product quality, reducing production cost, and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment , example 1
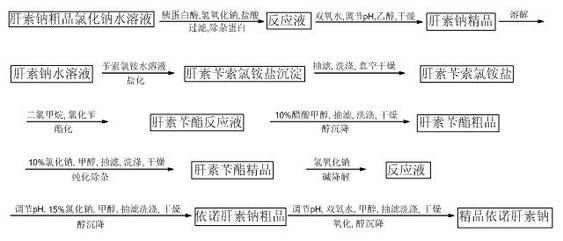
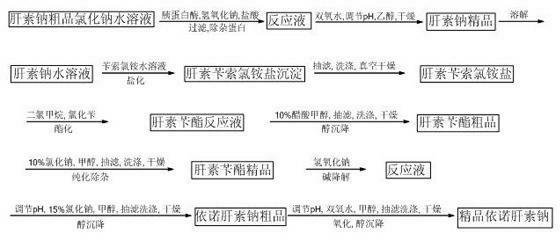
[0024] Weigh 20 g of crude bovine lung heparin sodium for later use.
[0025] 1. Preparation of high-quality heparin sodium:
[0026] At room temperature, 1 part by weight of crude bovine heparin sodium was dissolved in 10 parts by weight of 3% sodium chloride solution, and suction filtered to remove insoluble impurities. Add 2-3% by weight of trypsin to the above filtrate, adjust the pH to 7-9 with 2 mol / L sodium hydroxide solution, react in a water bath at 40°C for 5 hours, adjust the pH to 1.5-2.0 with 2 mol / L hydrochloric acid, filter , to remove protein. Add 2% to 3% hydrogen peroxide by weight of crude heparin sodium to the above clear liquid, adjust the pH to 8.5 to 9.5 with 2mol / L sodium hydroxide solution, oxidize in a constant temperature water bath at 25°C for 15 hours, filter with suction, and add to the filtrate 3-4 times the volume of ethanol of the filtrate, precipitate for 12 hours, filter the supernatant to obtain a precipitate. The precipitate was ground a...
specific Embodiment , example 2
[0037] The difference between this example and Example 1 is that the crude heparin sodium is 200g. Referring to the steps described in Example 1, the titer of the obtained refined heparin sodium is 138IU / mg; the average molecular weight of the refined enoxaparin sodium is 4327, and the molecular weight is less than 2000 Daltons accounted for 17.8%, polysaccharide chain molecular weight of 2000-8000 Daltons accounted for 72.5%, anti-Xa biological activity was 108.0 IU / mg, anti-Ⅱa biological activity was 25.7 IU / mg, and the ratio of anti-Xa / anti-Ⅱa was 4.20. .
specific Embodiment , example 3
[0039] The difference between this example and Example 1 is that the crude heparin sodium is 1 kg. Referring to the steps described in Example 1, the measured potency of the obtained heparin sodium product is 147 IU / mg; the average molecular weight of the enoxaparin sodium product is 4560, and the molecular weight is less than 2000 Daltons accounted for 17.3%, polysaccharide chain molecular weight of 2000-8000 Daltons accounted for 71.7%, anti-Xa biological activity was 106.3 IU / mg, anti-Ⅱa biological activity was 25.8 IU / mg, and the ratio of anti-Xa / anti-Ⅱa was 4.12.
[0040] The product of the present invention uses crude heparin sodium prepared from fresh bovine lungs in halal slaughterhouses as a starting material, is suitable for sale and use in Southeast Asia, the Middle East and Muslim countries, and is a product highly demanded by Muslim countries and the world's pharmaceutical industry.
[0041] The manufacturing process of the present invention adopts the crude hepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com