Enoxaparin sodium and production purification method thereof
A technology of enoxaparin sodium and purification method, applied in the field of enoxaparin sodium and its production and purification, can solve problems such as thrombosis mortality, and achieve the effects of improving product quality, simplifying production process and achieving good quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment , example 1
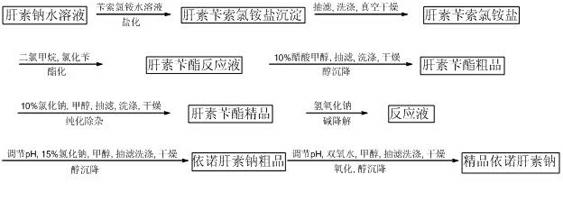
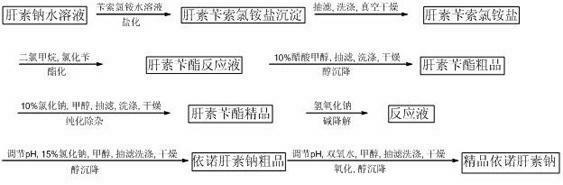
[0018] Weigh 72g heparin sodium, 180g benzethonium chloride, and 240g benzyl chloride for later use.
[0019] 1. Preparation of heparin benzethonium chloride salt:
[0020] At room temperature, 1 part by weight of heparin sodium was dissolved in 15 parts by weight of deionized water, that is, 72 g of heparin sodium was dissolved in 1080 g of deionized water, 180 g of benzethonium chloride was dissolved in 5.5 parts by weight of deionized water and mixed Stir evenly, add the benzethonium chloride aqueous solution to the heparin sodium aqueous solution, and carry out a sufficient salinization reaction. The reaction time is 1 hour, and the obtained heparin benzethonium chloride salt is left to stand, vacuum filtered, and the solid is obtained after suction filtration , and then the obtained solid was stirred and washed with deionized water and then suction-filtered. After washing and suction-filtering twice in this way, a new solid was obtained, and the new solid was dried at 40-...
specific Embodiment , example 2
[0030] The differences between this example and Example 1 are 200 g of heparin sodium, 500 g of benzethonium chloride, and 667 g of benzyl chloride.
specific Embodiment , example 3
[0032] The difference between this example and Example 1 is that heparin sodium is 1kg, benzethonium chloride is 2.5g, and benzyl chloride is 3.34kg.
[0033] The present invention can effectively reduce production cost, improve product quality, and simplify the production process. According to the production process, the finally obtained high-quality enoxaparin sodium is a mixture of different types, which meets the quality standards of the European Pharmacopoeia, and the yield is equivalent to that of foreign countries. The quality is better than similar products currently on the market.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com