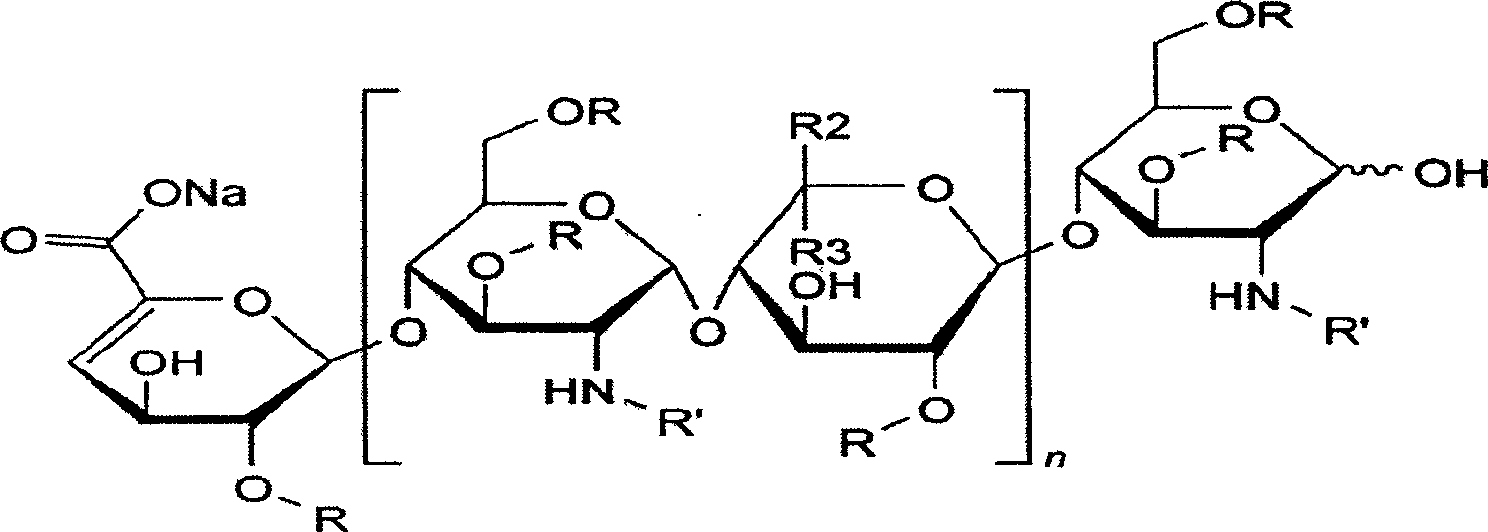
Production method for purifying enoxaparin sodium
A technology of enoxaparin sodium and its production method, which is applied in the direction of chemical instruments and methods, other chemical processes, etc., can solve the problems that cannot be achieved, residual pigments cannot be further separated, and affect the quality of enoxaparin finished products, so as to achieve easy industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the preparation of enoxaparin sodium
[0031] 1.1 Salification of heparin with long-chain quaternary ammonium salts in aqueous media
[0032] Weigh 100g of heparin raw material and 20g of sodium chloride and dissolve in 1L of water, slowly add 800ml of 95% ethanol at room temperature, after precipitation overnight, remove the supernatant, add 1L of water to dissolve the precipitation. Weigh 250 g of benzylethylammonium and dissolve it in 1 L of water, slowly add it into the heparin solution, and stir. The precipitate was filtered, washed with water, filtered and dried to obtain about 327 g of heparin sodium quaternary ammonium salt.
[0033] 1.2 Benzyl esterified heparin
[0034] The above heparin sodium quaternary ammonium salt was dissolved in 1.2L of dichloromethane, 350ml of benzyl chloride was slowly added, and stirred at 35°C for 25 hours. In addition, 200g of sodium acetate was weighed and dissolved in 2L of methanol, slowly added to the above di...
Embodiment 2
[0037] Embodiment 2, decolorization method one
[0038] Weigh 23 grams of crude enoxaparin, add water to dissolve, and the colorimetry is equivalent to the yellow No. 3 color specified in European Pharmacopoeia 4. Add activated carbon, stir at 100rpm for 40 minutes, filter with filter paper to remove activated carbon, and then filter with microporous membrane to remove residual activated carbon. Put the enoxaparin sodium solution on a H103 non-polar macroporous polystyrene adsorption resin column (manufactured by Nankai University Chemical Factory), with a column bed volume of 150 ml, and elute with water to collect the target peak. The collected solution was eluted twice on the column to collect the target peak. The colorimetry was better than the yellow No. 5 color specified in European Pharmacopoeia 4, and close to the yellow No. 6 color. The collected solution was precipitated with ethanol, filtered, freeze-dried, and weighed to obtain 18.8 g. After testing, the indicato...
Embodiment 3
[0044] Embodiment 3: decolorization method two
[0045] Weigh 30 grams of enoxaparin crude product, add water to dissolve, and the colorimetry is close to the yellow No. 3 color specified in European Pharmacopoeia 4. Put the enoxaparin solution on an XAD-7HP polar macroporous polyalkylene ester adsorption resin column (manufactured by Rohm Haas), with a column bed volume of 120 ml, and elute with water to collect the target peak. The column was eluted for 2 more times, and the target peak was collected, and the colorimetry was yellow No. 4 color defined in European Pharmacopoeia 4. Add activated carbon, stir at 100rpm for 40 minutes, filter with filter paper to remove activated carbon, and then filter with microporous membrane to remove residual activated carbon. The colorimetry is better than the yellow No. 5 color specified in European Pharmacopoeia 4. The collected solution was precipitated by ethanol, filtered, freeze-dried, and weighed to obtain 26.4 grams. After testin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com