Synthesis and applications of an albumin bounding type 5-fluorouracil prodrug
A technology of albumin-binding and fluorouracil, which is applied in drug combinations, medical preparations containing active ingredients, anti-tumor drugs, etc., to prolong the plasma half-life and improve anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
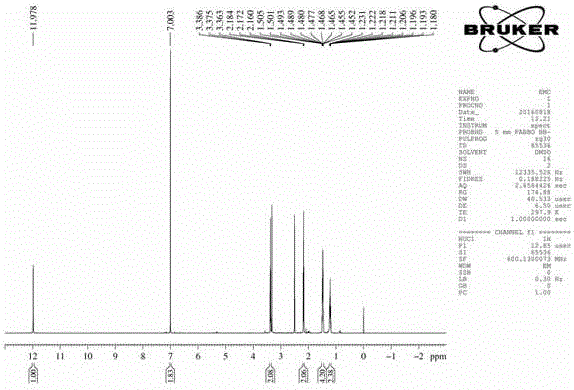
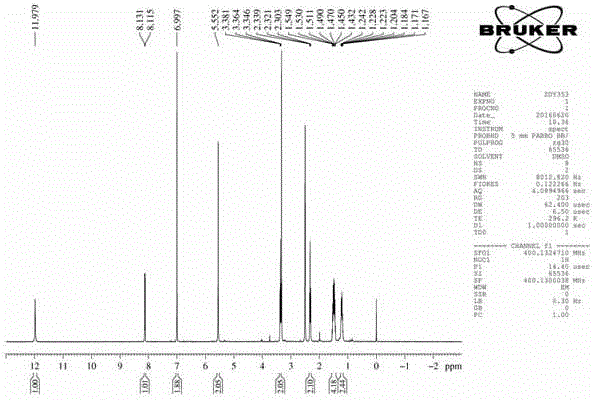
[0035] Preparation of 6-maleimidocaproic acid and 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil
[0036] (a) Dissolve 7.845g (80mmol) of maleic anhydride and 10.494g (80mmol) of 6-aminocaproic acid in 70mL of glacial acetic acid, reflux in an oil bath at 140°C for about 2.5h, spin to dry the solvent after the reaction, and remove the toluene with water (2×30mL), spin-dried and then extracted with ethyl acetate, the organic layers were combined and dried overnight with anhydrous sodium sulfate, spin-dried, separated and purified on a silica gel column to obtain the product (II).
[0037] (b) Take 4.215g (32.4mmol) of 5-fluorouracil, add 5.357mL (71.28mmol) of 37% formaldehyde solution, put in an oil bath at 55°C until the solid is completely dissolved, continue to react for about 4 hours, and spin dry to obtain a colorless transparent viscous liquid (Ⅲ). Another 5.699g (27mmol) of 6-maleimidocaproic acid, 7.421mL (67.5mmol) of N-methylmorpholine and 13.346g (35.1mmol) of HATU...
Embodiment 2
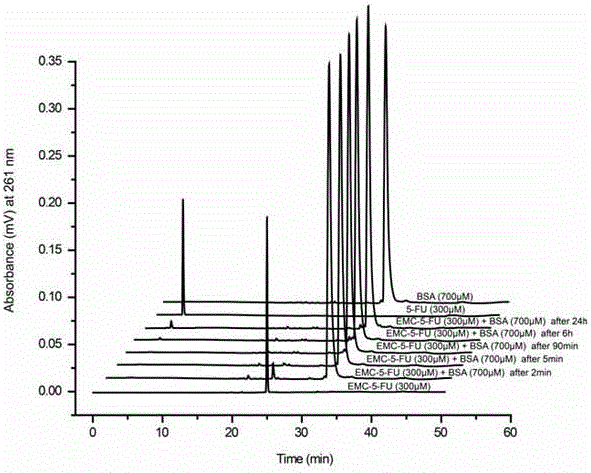
[0043] Binding of 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil to bovine serum albumin in vitro
[0044] Weigh 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil and dissolve it in pH 7.4 phosphate buffer solution to make the concentration 300 μM. Another bovine serum albumin with a mass of 46.2 mg was weighed, and 1 mL of 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil solution was added, shaken slightly to dissolve, so that the concentration of bovine serum albumin 700 μM. The mixed solution was incubated in a constant temperature shaker at 37°C, samples were taken at 2, 5, 90 minutes and 6, 24 hours, and 10 μM samples were injected into high performance liquid chromatography.
[0045] In addition, a group of control experiments were carried out. First, the 34 free sulfhydryl groups of bovine serum albumin were fully blocked with excess 6-maleimidocaproic acid, and then mixed with 1-(6-maleimidocaproyl Oxymethyl)-5-fluorouracil was incubated for binding.
[0046] The results...
Embodiment 3
[0048] 5-fluorouracil release experiment of 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil albumin complex in different pH phosphate buffer
[0049] The reaction conditions of the in vitro binding experiment were adopted, that is, 46.2 mg of bovine serum albumin was weighed and added to 1 mL of 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil aqueous solution with a concentration of 300 μM at 37 ° C. Incubate under the conditions for 90 minutes, at this time, no free 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil can be detected, that is, the reaction is complete. After lyophilization, 1-(6-maleimidocaproyloxymethyl)-5-fluorouracil albumin complex is obtained. Take a portion of the above freeze-dried sample, add it to 1 mL of phosphate buffer (pH 2.5, 7.4 and 10.5), transfer the solution to a dialysis bag, place in a conical flask containing 10 mL of the corresponding phosphate buffer, The hydrolysis experiments were carried out in a constant temperature shaker at 37°C. Draw 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com