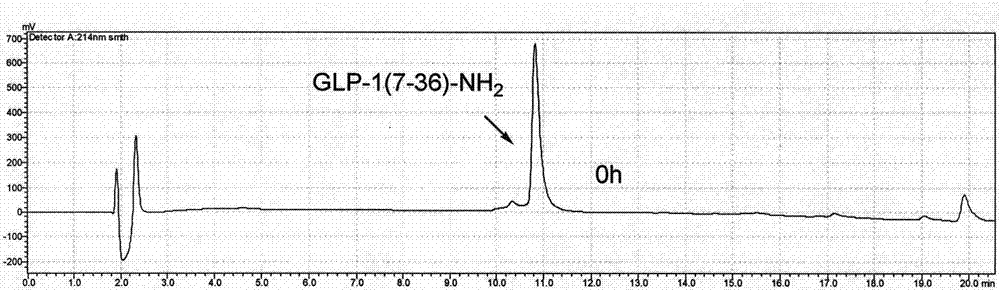
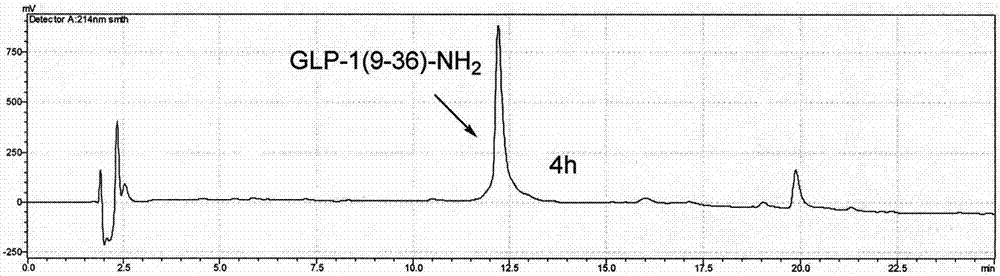
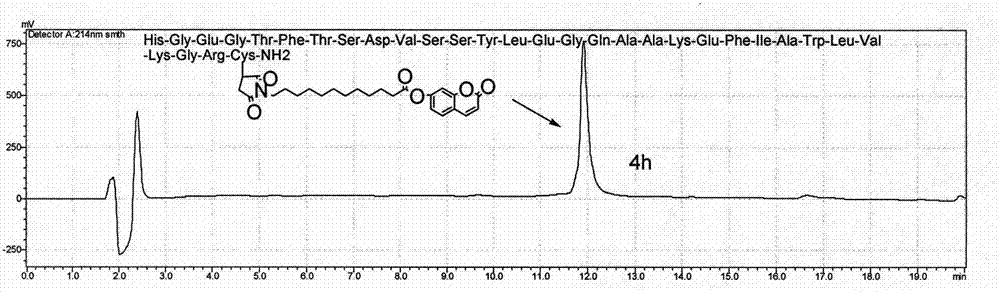
Novel long-acting glucagon-like peptide 1 (GLP-1) analogues and application thereof
A glucagon and GLP-1 technology, applied in the field of novel long-acting glucagon-like peptide-1 analogs, can solve the problems of loss of biological activity of GLP-1, loss of histidine residues, etc. Significant effect, hypoglycemic effect, effect of long plasma half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102]
[0103] microwave-facilitated solid-phase synthesis
[0104] (1) Small molecule modified cysteine synthesis
[0105] Weigh 0.21g of Fmoc-Cys-OH, dissolve it in DCM, add 0.18g 7-(6-maleimidopropionyl)-7-hydroxycoumarin, 8ul DIEA as a catalyst, and stir at room temperature After reacting for 4 hours, after the reaction was completed by TLC monitoring, the reaction solution was concentrated under reduced pressure and separated by column chromatography to obtain 0.33 g of the product, with a yield of 83%.
[0106] MS (70eV) m / z: 720.5 ([M+Na] + ).
[0107] (2) Swelling of the resin
[0108] Weigh 50 mg of Fmoc-Rink amide-MBHA Resin (substitution amount 0.4 mmol / g), swell with 7 mL of DCM for 30 min, filter to remove DCM, then swell with 10 mL of NMP for 30 min, and finally rinse with NMP, DCM, and 7 mL of NMP respectively.
[0109] (3) Microwave promotes removal of Fmoc protecting group
[0110] Put the swollen resin into the reactor, add 7mL of 25% piperidine / NM...
Embodiment 2~16
[0124] According to the general method described in Example 1, the glucagon-like peptide-1 (GLP-1) analogs of Examples 2 to 16 were synthesized according to the corresponding sequences, and the respective analogues were confirmed by electrospray mass spectrometry (ESI-MS). molecular weight.
Embodiment 2
[0126]
[0127] The theoretical relative molecular mass is 3668.8. ESI-MS m / z: found[M+4H] 4+ 918.2, [M+5H] 5+ 734.7;calu[M+4H] 4+ 917.9, [M+5H] 5+ 734.3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com