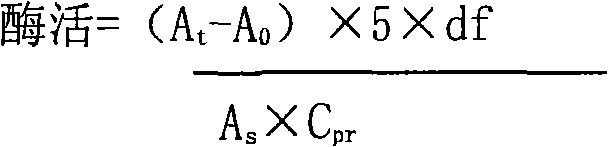Polyethylene glycol modified L-Asparaginasum and modification method thereof
A technology of asparaginase and polyethylene glycol, applied in the direction of hydrolase, etc., can solve the problems of inhomogeneous modification products, difficulty in separation of modified mixtures, prolongation of half-life of biological activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 polyethylene glycol modified asparaginase
[0037] Take 100ml of asparaginase stock solution (buffer system is pH7.0, 20mMPB buffer, protein concentration is 5mg / ml), and another 100mg of mPEG-ALD (20kd), add it to the asparaginase stock solution, stir at room temperature to make it Dissolve rapidly, and stir slowly at 10°C for 8 hours. Then add 100mg of mPEG-ALD (20kd), stir slowly at 10°C for 8 hours, and purify the modification by gel exclusion chromatography and ion exchange chromatography.
Embodiment 2
[0038] Embodiment 2 SDS-PADE electrophoresis detection
[0039] Electrophoresis instrument: DYY-6C
[0040] Stacking Gel: 5%
[0041] Separating gel: 12%
[0042] Loading volume: 25μl
[0043] Voltage setting: 80V, 30min; 180V, 60min
[0044] Test results: After analysis by the gel imaging system, the total modification efficiency of polyethylene glycol is 76.25%, and the modification efficiency of the main band is 63.41%. The molecular weight of the main band is 81400 Daltons, while the theoretical molecular weight should be 75500 Daltons. This is because when polyethylene glycol migrates in polyacrylamide gel, polyethylene glycol looks like a long and flexible tail, which slows down the overall migration speed.
Embodiment 3
[0045] Example 3 Enzyme Activity Determination
[0046] Utilizes the Mashburn Wriston method. The method determines the hydrolysis rate of the substrate by asparaginase (Asparaginase) by measuring the concentration of amino acids in the product, and the amount of ammonia produced is determined by the color reaction between the Nessler reagent and the protein.
[0047]
[0048] A t : sample value
[0049] A 0 : negative control value
[0050] df: dilution factor
[0051] A s : positive control value
[0052] C pr : protein sample concentration
[0053]
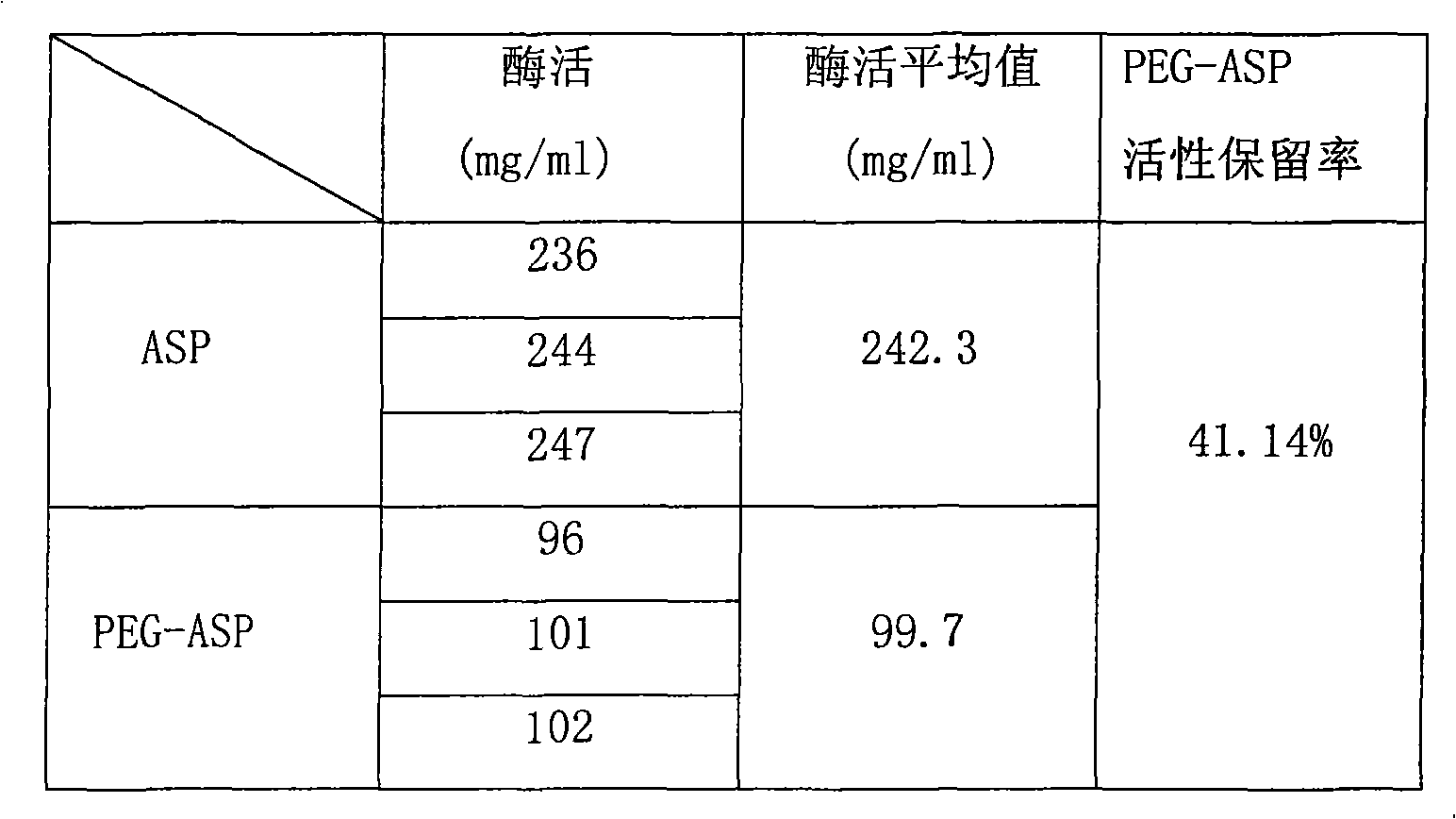
[0054] Asparaginase modified by two polyethylene glycols has an enzyme activity retention rate of 41.14%, and the enzyme activity has not decreased due to the connection of the second polyethylene glycol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com