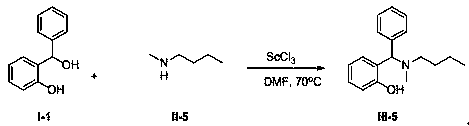
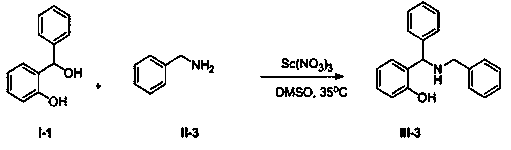
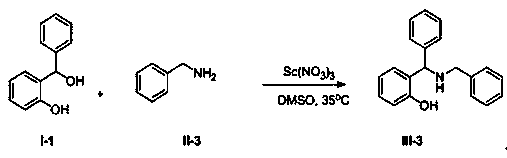
Synthetic method of Betti base derivative
A synthesis method and a technology of derivatives, which are applied in the field of synthesis of Bety base derivatives, can solve problems such as cumbersome post-processing operations, and achieve the effects of avoiding expensive catalysts, high reaction yields, and simple operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Add 2.00 g (10 mmol) of compound I-1, 2.06 g (20 mmol) of compound II-1, and 0.49 g (1 mmol) of solid Sc(OTf) into a 100 mL round bottom flask 3, and finally 20 mL of dry 1,2-dichloroethane was added, and the resulting mixture was stirred at 80° C. for 10 hours. After the reaction mixture was cooled to room temperature, it was poured into ice water, extracted with 50mL×3 methylene chloride, and the extracted organic phases were combined, washed once with saturated brine, anhydrous Na 2 SO 4 Dry, concentrate and remove the solvent to obtain the crude product, which is separated by column chromatography to obtain the pure product of compound III-1. Oily liquid, 2.62g, yield 92%. 1 H NMR (400 MHz, CDCl 3 ) δ : 11.72 (s, 1H), 7.36 (d, J = 7.1 Hz, 2H), 7.33~7.22 (m, 3H), 7.12~7.08 (m, 1H), 6.86(dd, J = 10.9 Hz, 4.2 Hz, 2H), 6.68 (td, J = 7.5 Hz, 0.9 Hz, 1H), 4.56 (s, 1H), 2.82~2.73 (m, 2H), 2.70~2.64 (m, 6H); 13 C NMR (CDCl 3 , 100MHz) δ : 156.69, 13...
Embodiment 2
[0038]
[0039] Add 2.00g (10mmol) compound I-1, 1.76g (20mmol) compound II-1, 0.98g (2mmol) solid Sc(OTf) into a 100mL round bottom flask 3 , and finally 50 mL of dry DMF was added, and the resulting mixture was stirred at 70° C. for 6 hours until the reaction was complete. The reaction mixture was cooled to room temperature, poured into water, stirred, extracted with 50mL×3 dichloromethane, combined and extracted organic phases, washed once with saturated brine, anhydrous Na 2 SO 4 Dry, concentrate and remove the solvent to obtain the crude product, which is separated by column chromatography to obtain the pure product of compound III-2. Oily liquid, 2.32g, yield 88%. 1 H NMR (400 MHz, CDCl 3 ) δ : 11.71 (s, 1H), 7.41 (d, J = 7.1 Hz, 2H), 7.30~7.21 (m, 3H), 7.13~7.09 (m, 1H), 6.94~6.92 (m, 1H), 6.86 (d, J = 8.1 Hz, 1H), 6.71 (td, J = 7.5 Hz, 0.9 Hz, 1H), 4.39 (s, 1H), 3.77~3.72 (m, 4H), 2.58~2.42 (m, 4H); 13 C NMR (CDCl 3 , 100MHz) δ : 156.15, 139.33, 129.43,...
Embodiment 3
[0041]
[0042] Add 2.00g (10mmol) compound I-1, 1.61g (15mmol) compound II-3, 0.12g (0.5mmol) solid Sc(NO 3 ) 3 , and finally 50 mL of dry DMSO was added, and the resulting mixture was stirred vigorously at 50 °C for 5 h. After the reaction mixture was cooled to room temperature, it was poured into water, stirred, extracted with 50mL×3 dichloromethane, combined and extracted organic phases were washed once with saturated brine, anhydrous Na 2 SO 4 Dry, concentrate and evaporate the solvent to obtain the crude product, and purify by column chromatography to obtain the pure product of compound III-3. Oily liquid, 2.37g, yield 90%. 1 H NMR (400 MHz, DMSO- d 6 )δ: 11.19 (s, 1H), 7.42 (d, J = 7.4 Hz, 2H), 7.35~7.30 (m, 6H), 7.27~7.21 (m, 2H), 7.08~7.04 (m, 2H), 6.73 (dd, J = 13.5Hz, 7.4Hz, 2H), 5.01(s, 1H), 3.66(s, 2H); 13 C NMR (DMSO- d 6 , 100 MHz) δ:156.85, 143.24, 140.07, 128.78, 128.71, 128.69, 128.34, 128.23, 127.97, 127.92, 127.41, 127.34, 119.29, 116.25, 62....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com