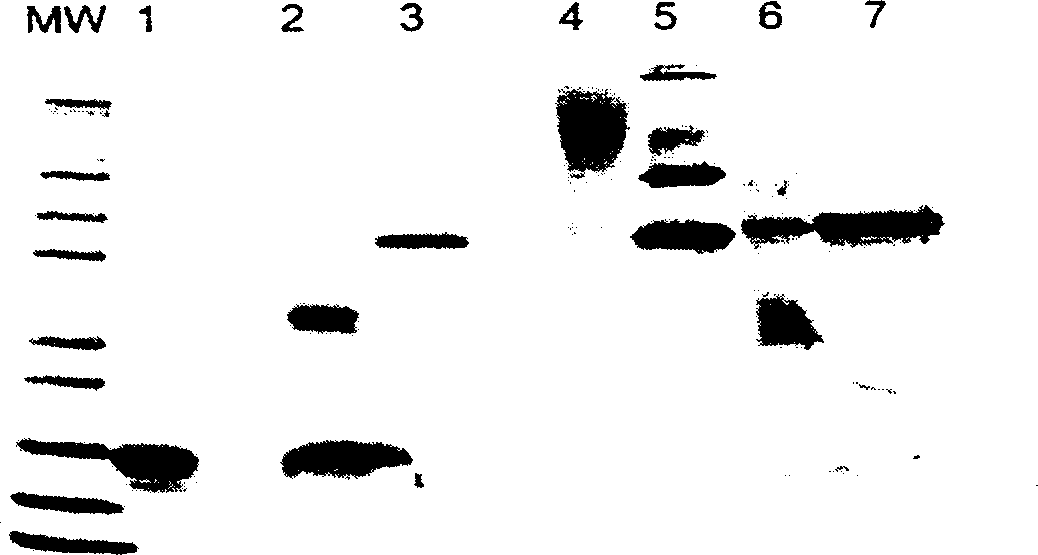
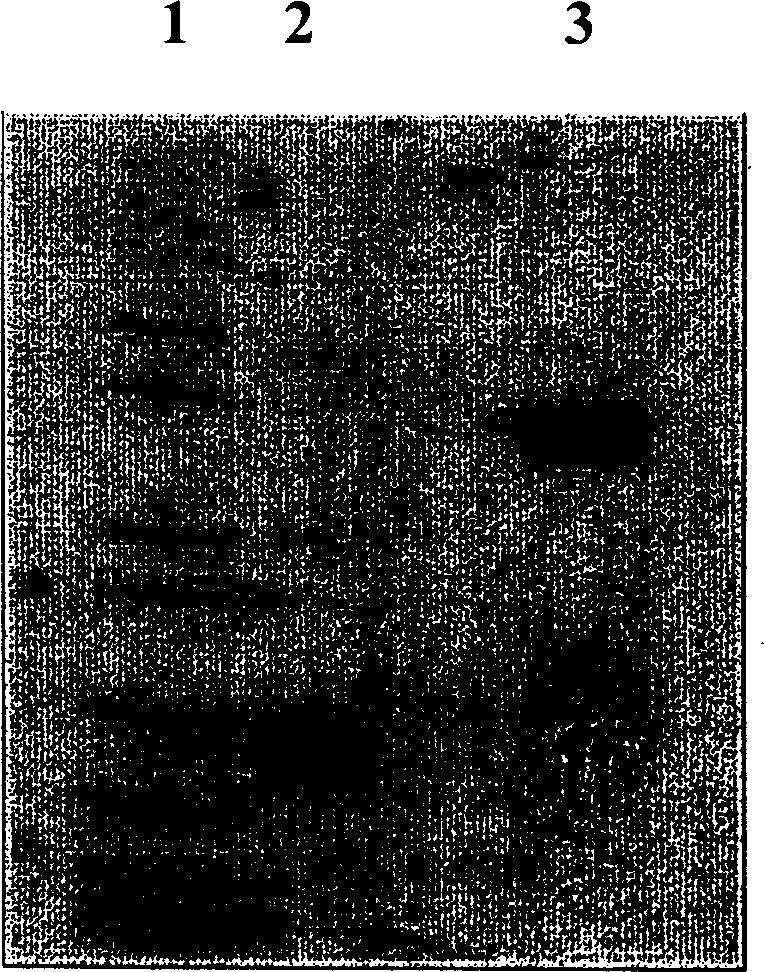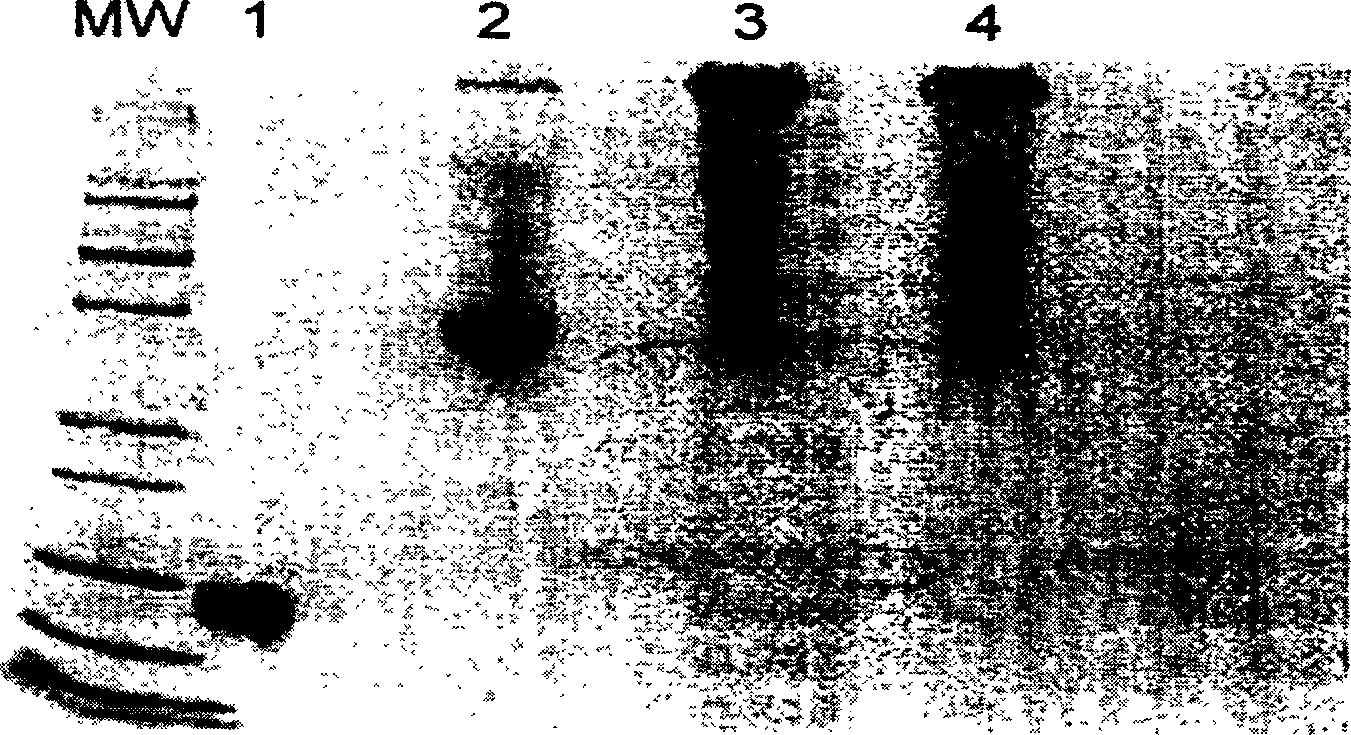Method for preparing polyethylene glycol-modified alpha-interferon 1b
A polyethylene glycol and interferon technology, applied in interferon, cytokines/lymphokines/interferon, chemical instruments and methods, etc., can solve problems such as shortened biological half-life, reduced product activity, and biological damage, and achieve The effect of simplifying the chemical reaction process and increasing the biological half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation of polyethylene glycol 12000 / 20000α-interferon 1b
[0066] Step (1) reaction
[0067] Dissolve 1.0 mg of α-interferon in 1 ml of 100 mM (pH 6.0) sodium phosphate buffer. Take 20 mg of SS-activated linear polyethylene glycol (SS-PEG12000) with a molecular weight of 12,000, quickly add it to the above solution, and shake it properly to completely dissolve the activated linear polyethylene glycol (SS-PEG12000) within 30 seconds. The reaction was carried out at 25°C for 60 minutes, and the reaction solution was rinsed without concentration, and could be further purified or subjected to the second step of reaction.
[0068] The pH range of the (1) step reaction is controlled at 6.0 to 6.5.
[0069] Step (2) reaction
[0070] Add 0.1 ml of 500 mM (pH 8.0) sodium phosphate buffer solution to the solution obtained in the above step 1. Put this solution in a water bath and preheat it to 25°C, and take another 20 mg of activated polyethylene glycol with ...
Embodiment 2
[0072] Example 2 Preparation of polyethylene glycol 20000 / 20000α-interferon 1b
[0073] The same steps as in Example 1 were used to prepare polyethylene glycol 20000 / 2000α-interferon 1b, except that: in the first step, SS-activated linear polyethylene glycol with a linear length range of 30 mg and 20,000 Daltons The diol is contacted with 1 mg of α-interferon 1b, and the length of the linear chain of polyethylene glycol is used for the reaction, that is, the mass ratio of α-interferon 1b to polyethylene glycol is in the range of 1:30.
[0074] In the second step, 30 mg of SS-activated linear polyethylene glycol with a linear length in the range of 20,000 Daltons is contacted with 1 mg of α-interferon 1b, and the reaction uses the length of the linear chain of polyethylene glycol, i.e., polyethylene glycol The mass ratio range of alcohol to α-interferon 1b is 1:30.
Embodiment 3
[0075] Example 3 Preparation of polyethylene glycol 12000 / 20000α-interferon 1b
[0076] Step (1) reaction
[0077] 1.0 mg of α-interferon 1b was dissolved in 1 ml of 100 mM (pH 6.0) sodium phosphate buffer. Take 30 mg of SC-activated linear polyethylene glycol (SC-PEG12000) with a molecular weight of 12,000, quickly add it to the above solution, and shake properly to completely dissolve the activated linear polyethylene glycol (SC-PEG12000) within 30 seconds. The reaction was carried out at 25°C for 60 minutes, and the reaction solution was rinsed without concentration, and could be further purified or subjected to the second step of reaction.
[0078] The pH range of the (1) step reaction is controlled at 6.0 to 6.5.
[0079] Step (2) reaction
[0080] Add 0.1 ml of 500 mM (pH 8.0) sodium phosphate buffer solution to the solution obtained in the above step 1. Put this solution in a water bath and preheat it to 25°C, and take another 20 mg of activated polyethylene glycol ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com