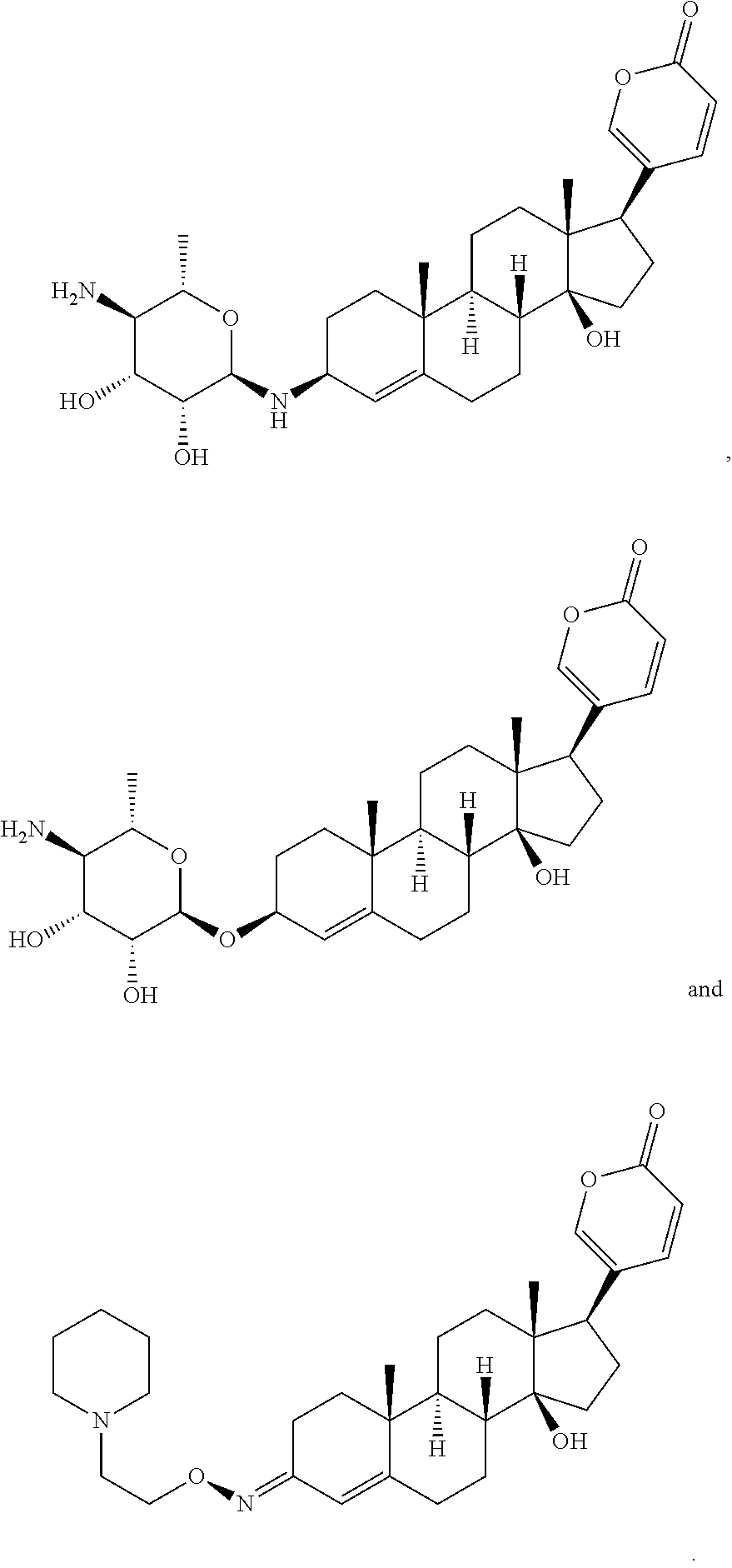
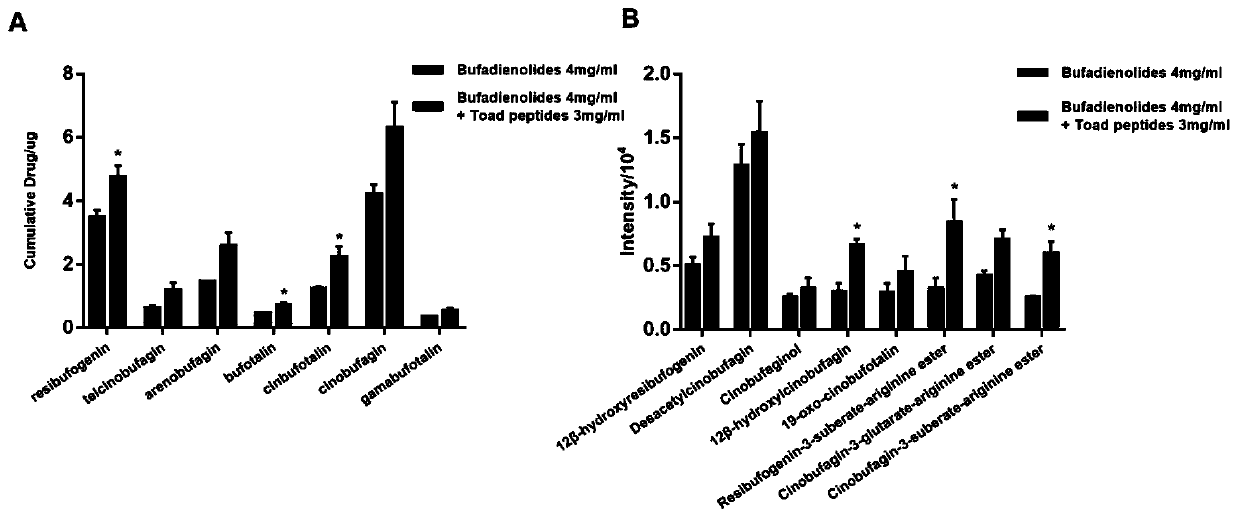
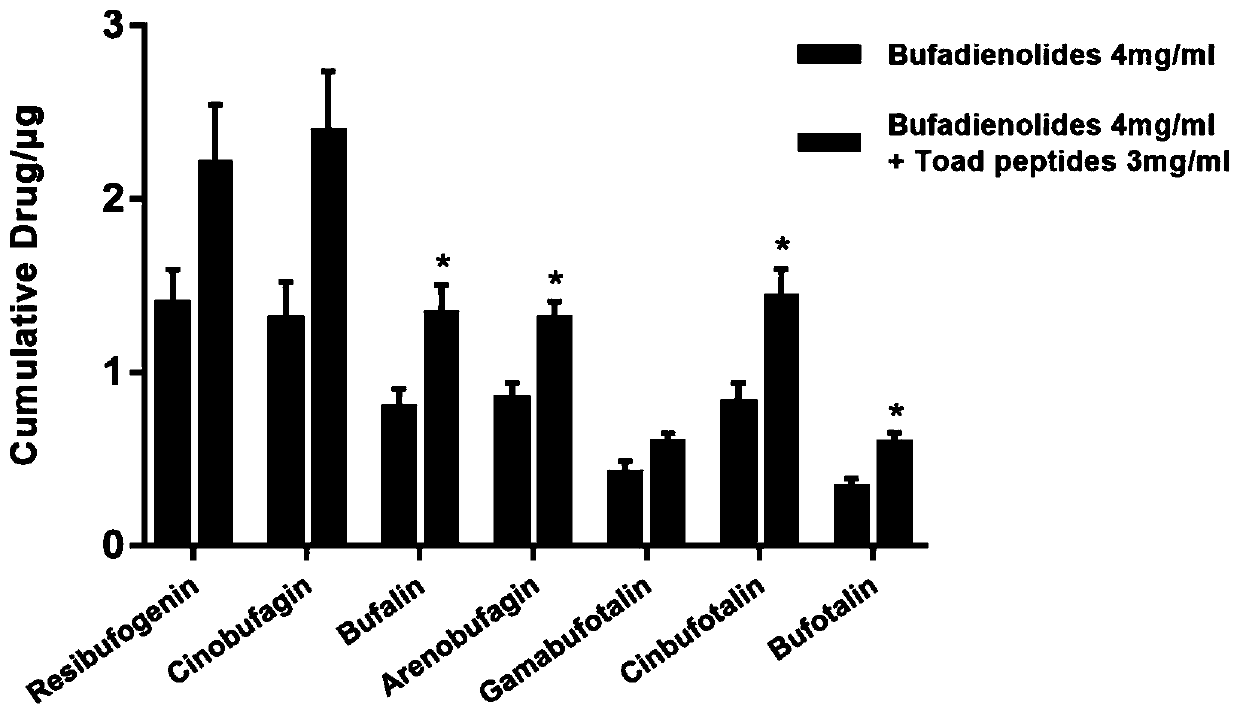
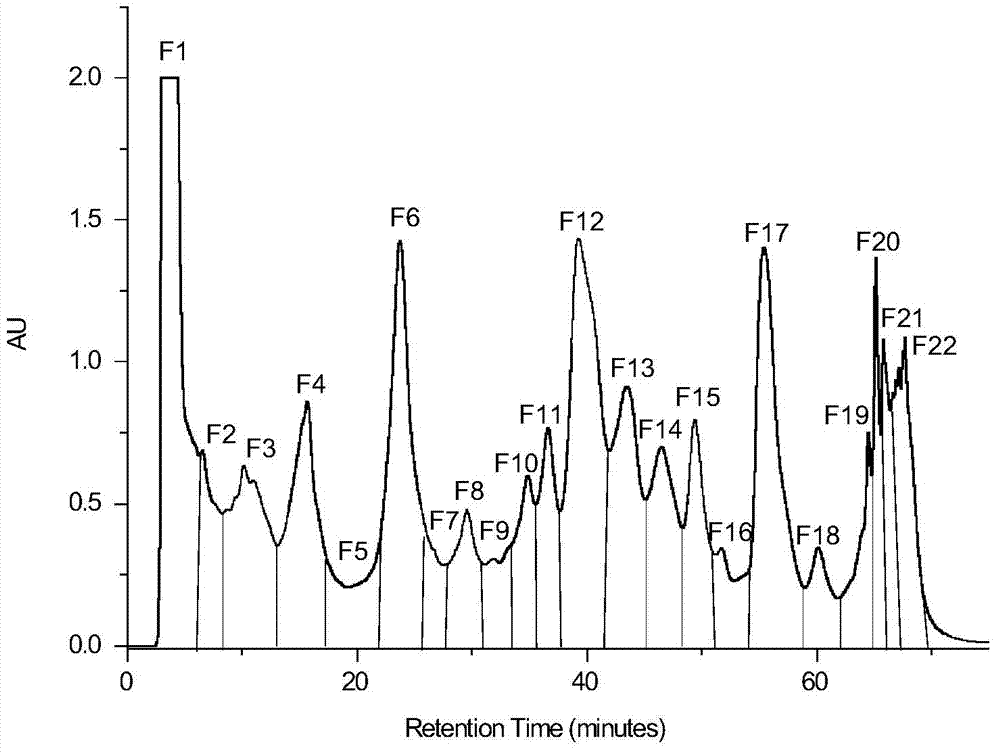
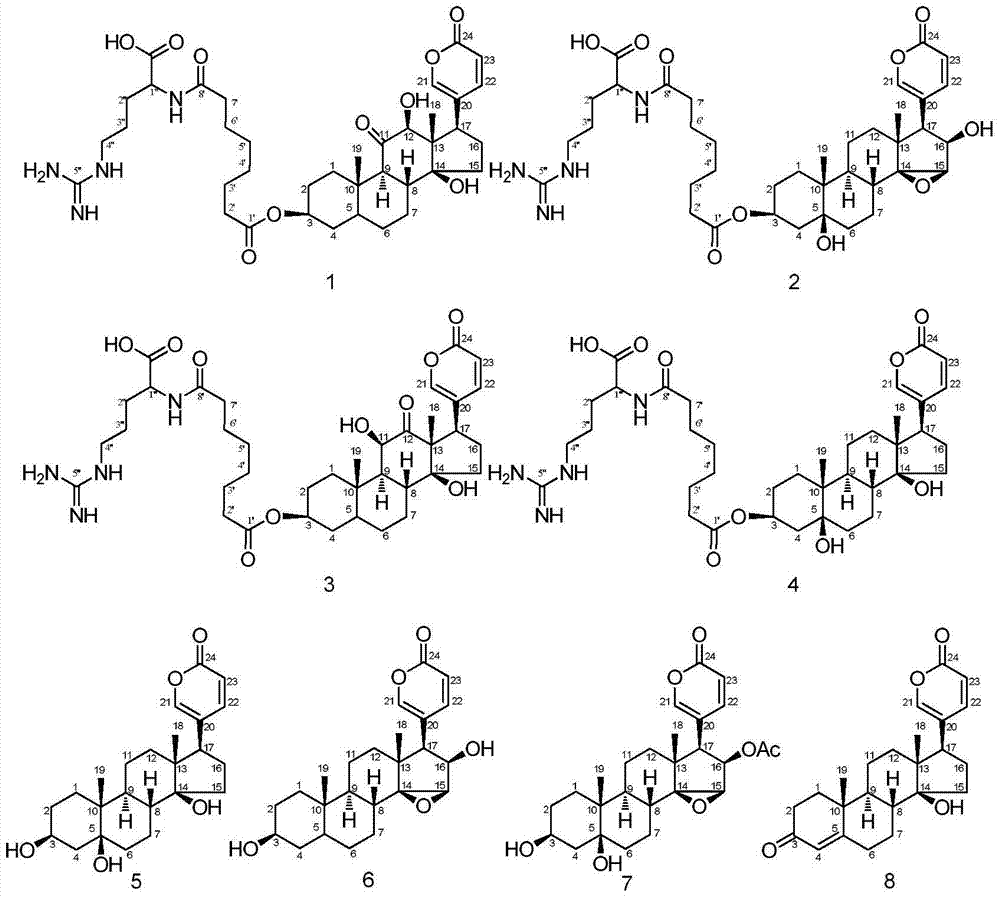
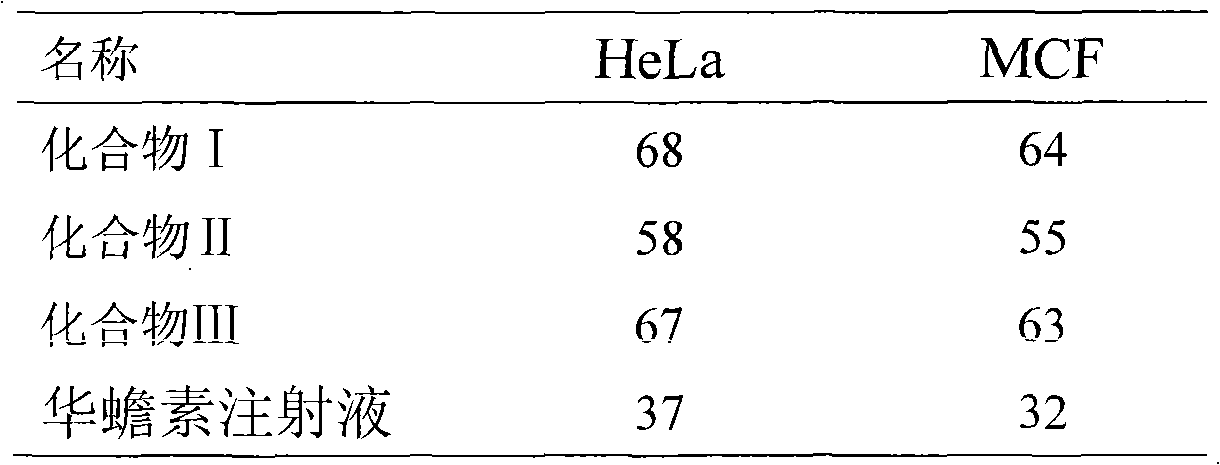
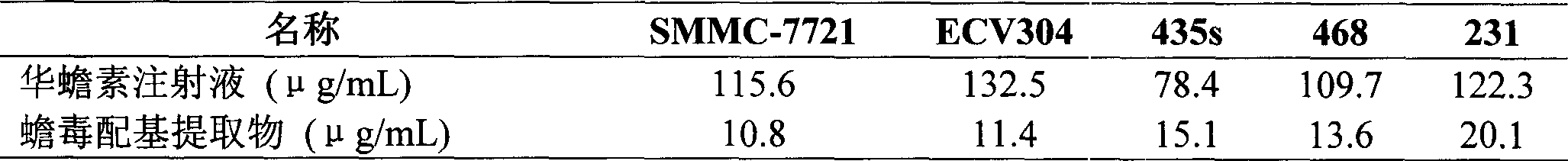
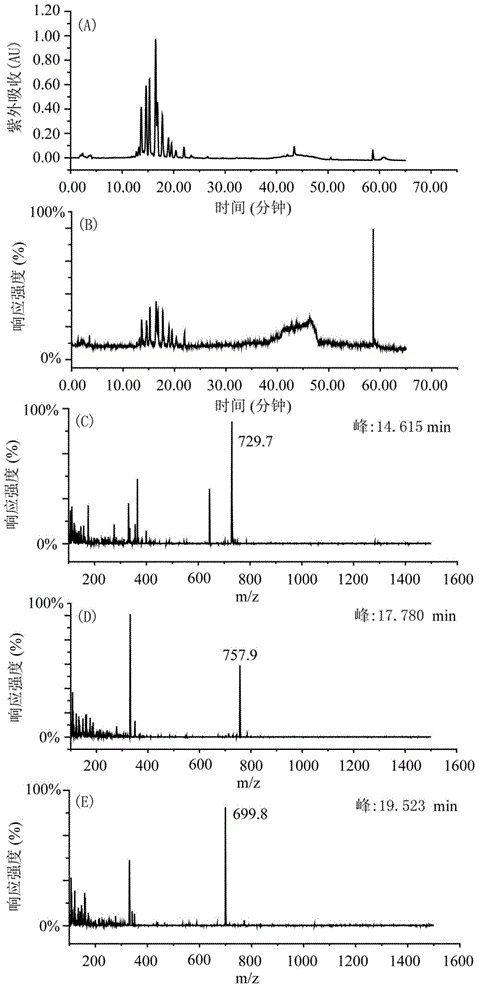
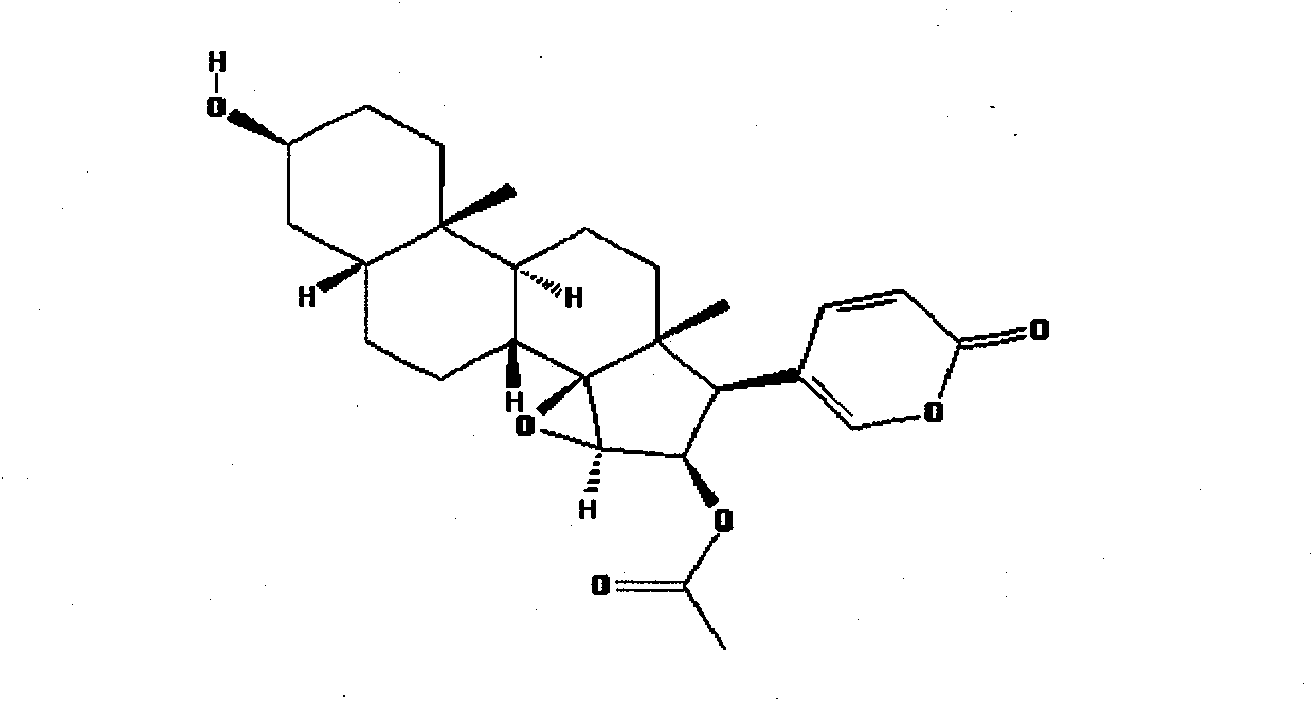
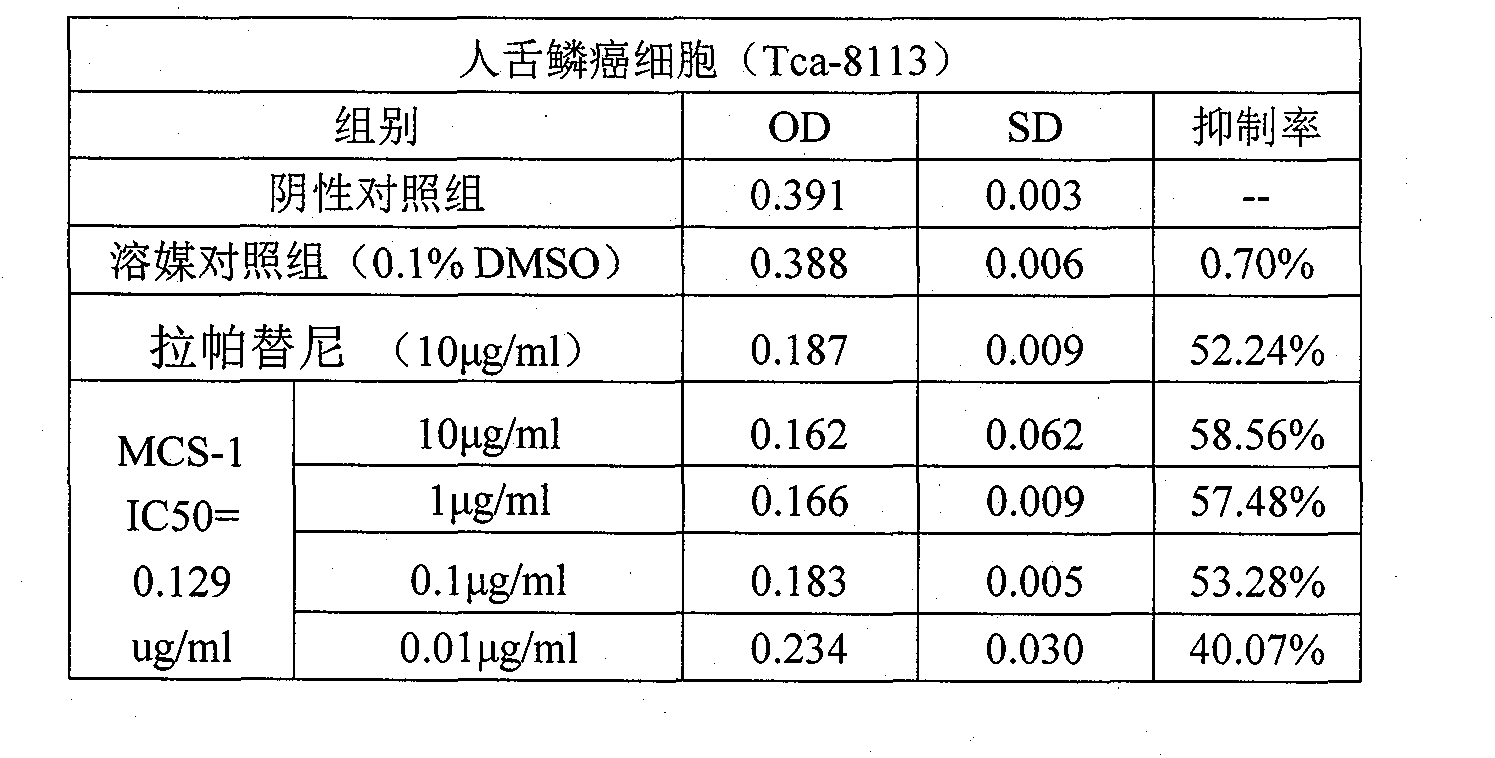
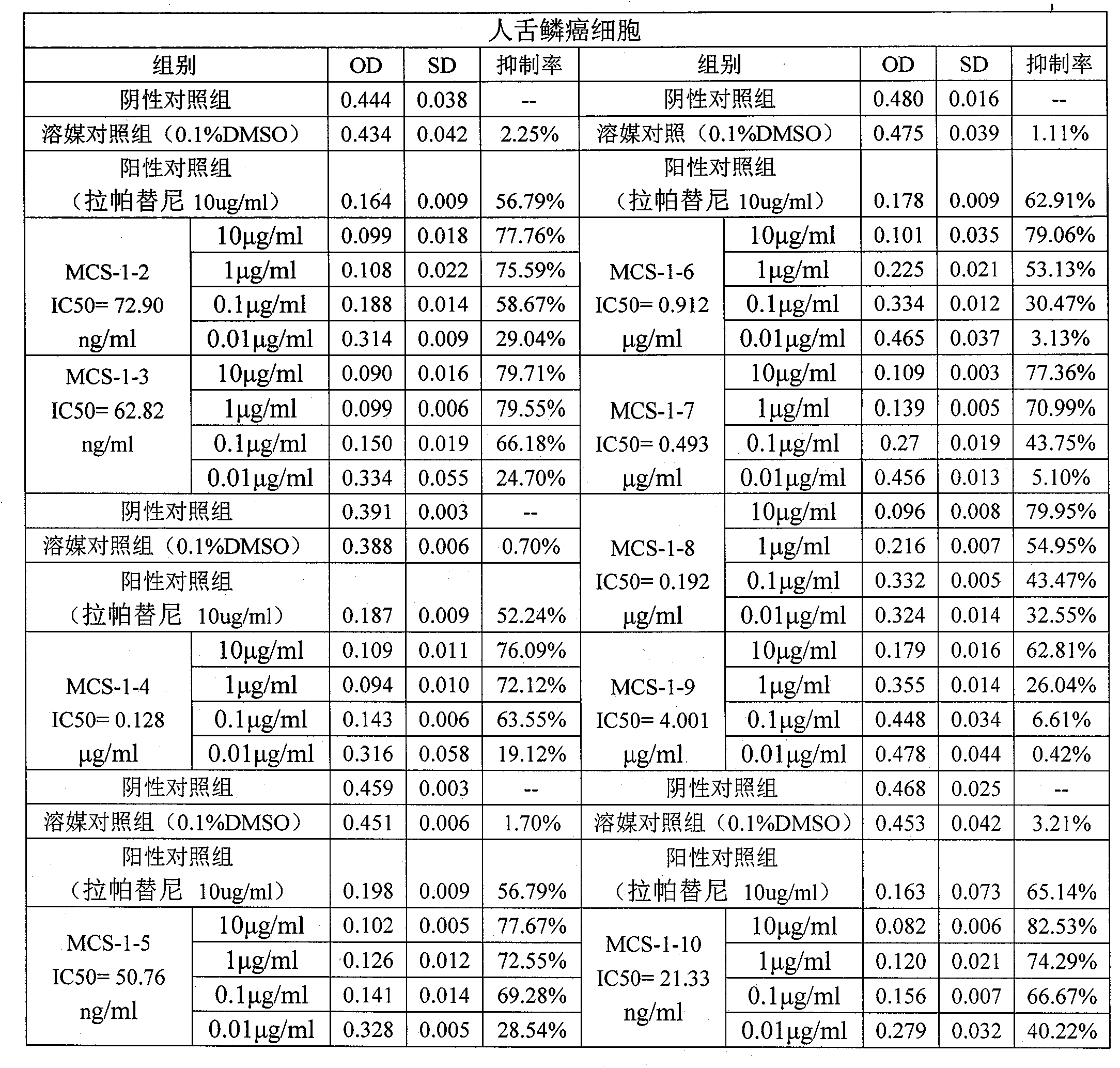
Patents
Literature
31 results about "Bufadienolide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
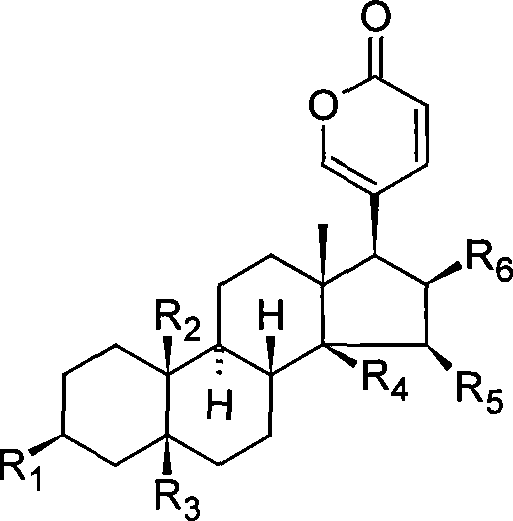
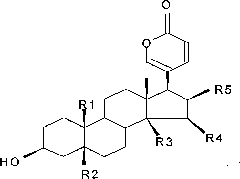
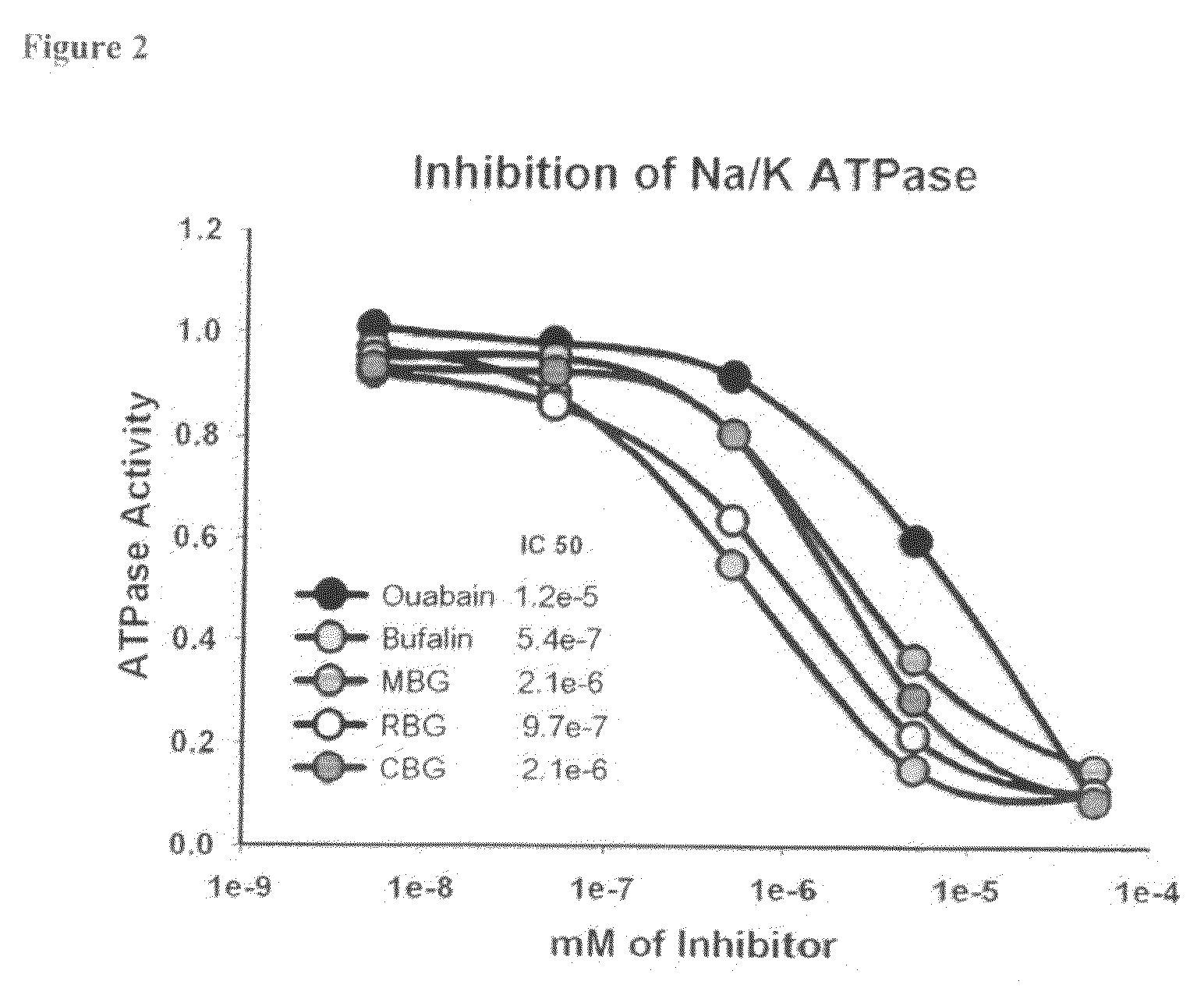
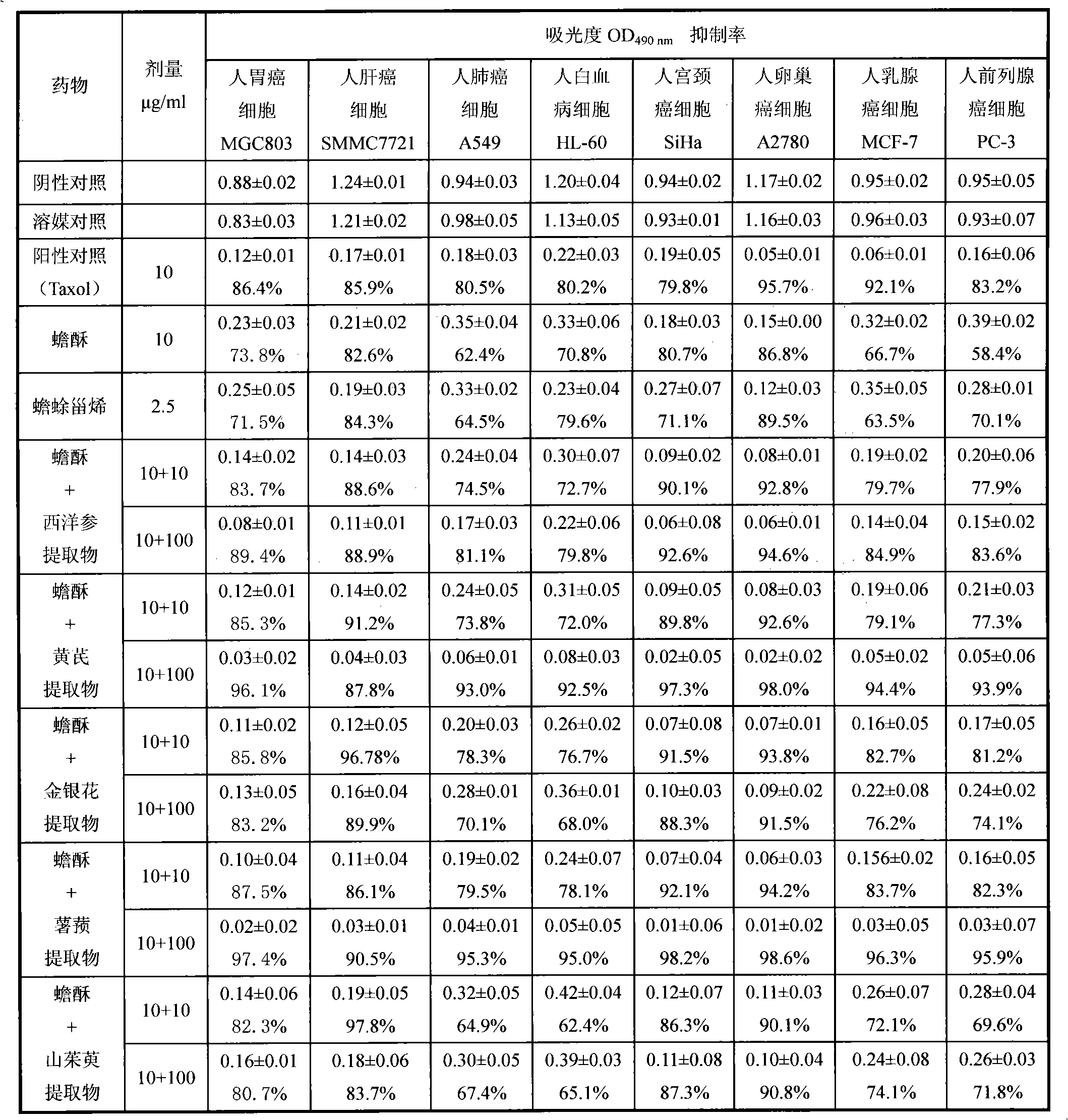
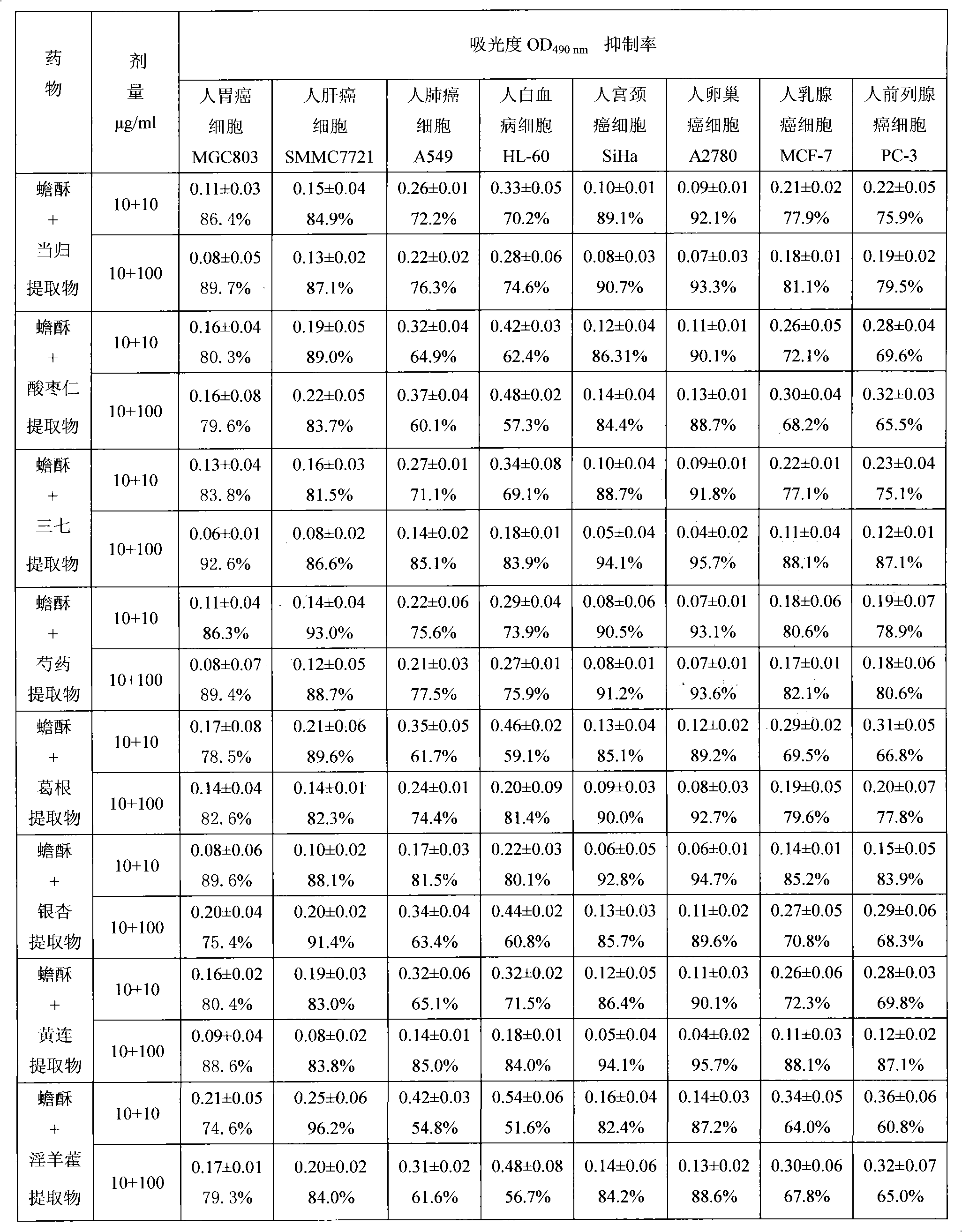
Bufadienolide is a chemical compound with steroid structure. Its derivatives are collectively known as bufadienolides, including many in the form of bufadienolide glycosides (bufadienolides that contain structural groups derived from sugars). These are a type of cardiac glycoside, the other being the cardenolide glycosides. Both bufadienolides and their glycosides are toxic; specifically, they can cause an atrioventricular block, bradycardia (slow heartbeat), ventricular tachycardia (a type of rapid heartbeat), and possibly lethal cardiac arrest.
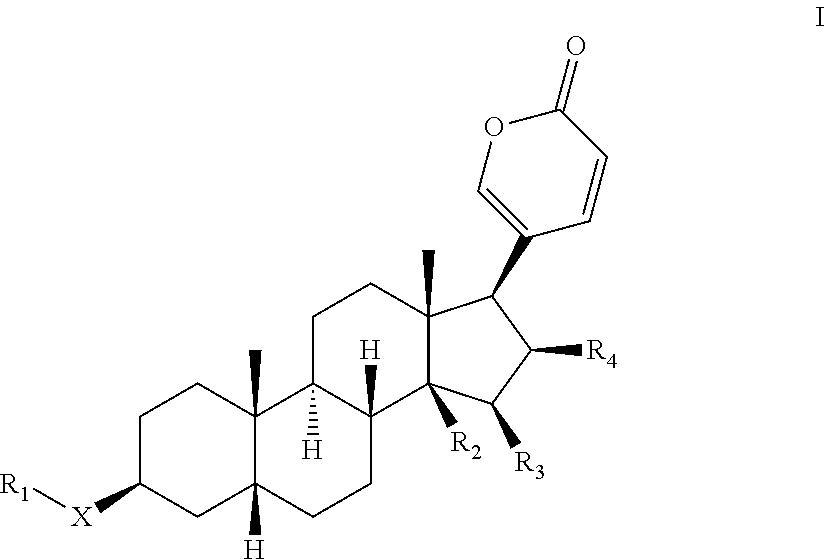
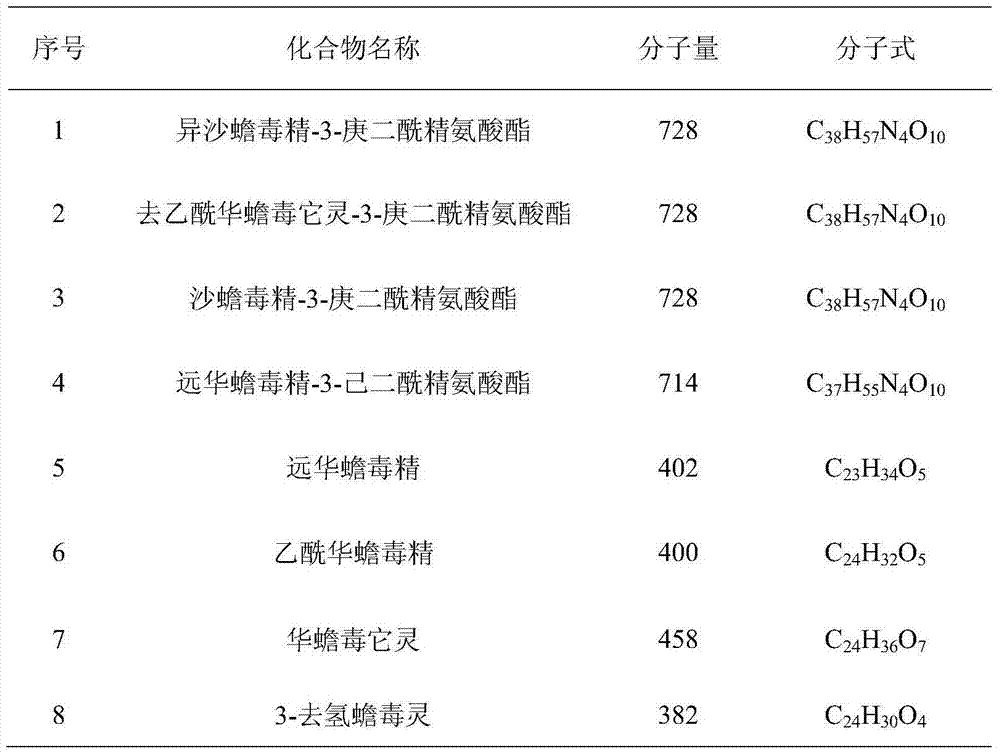
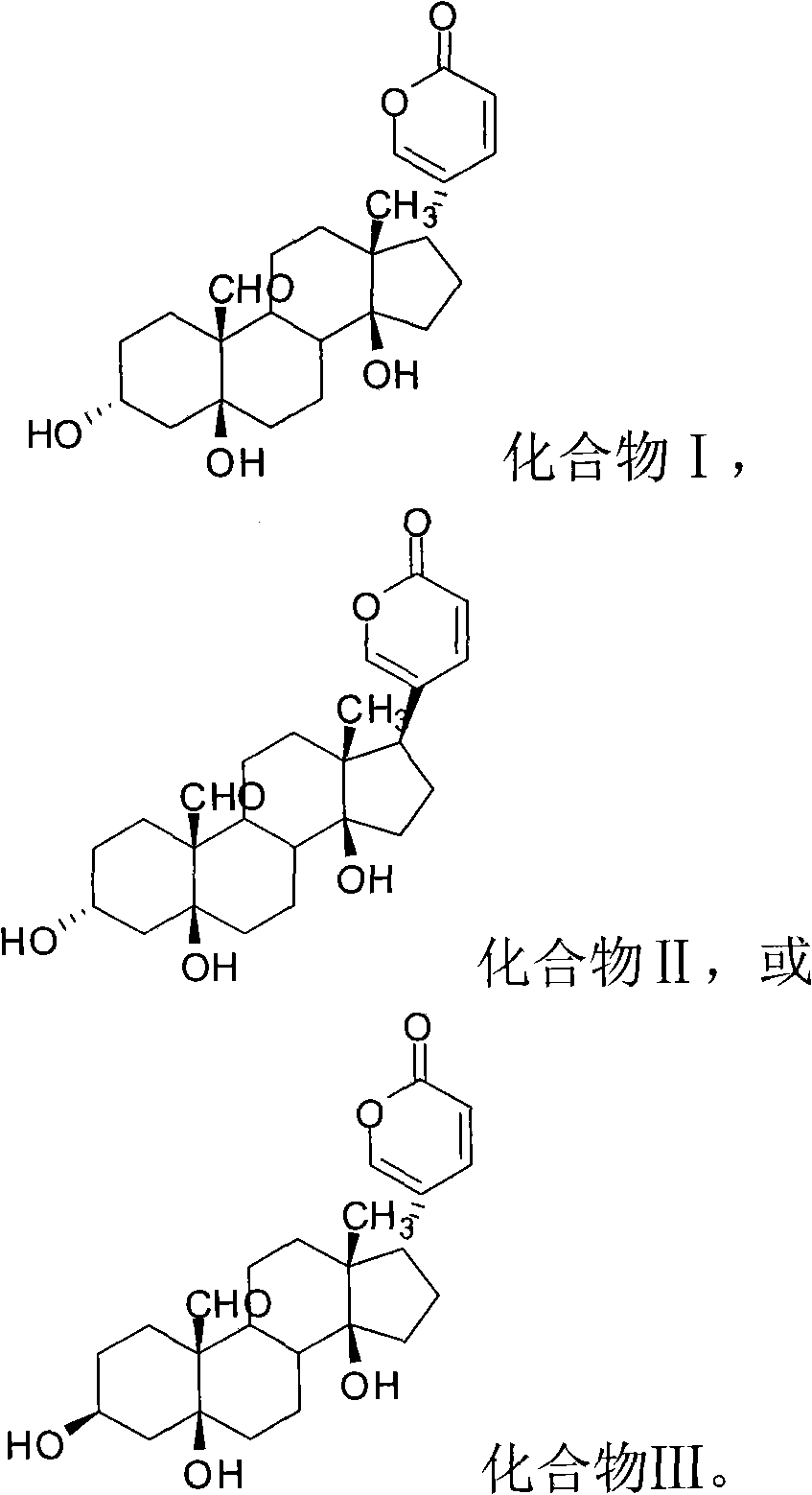
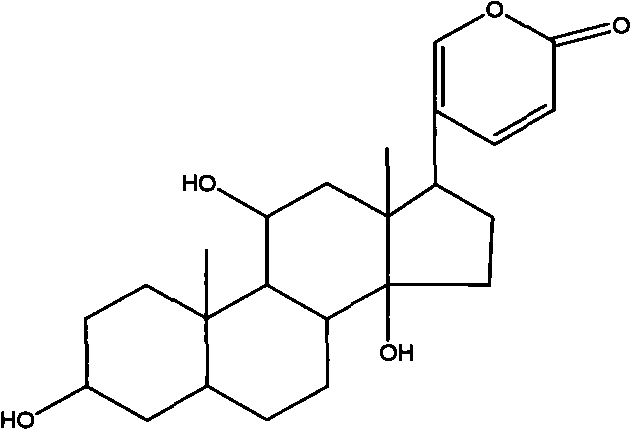
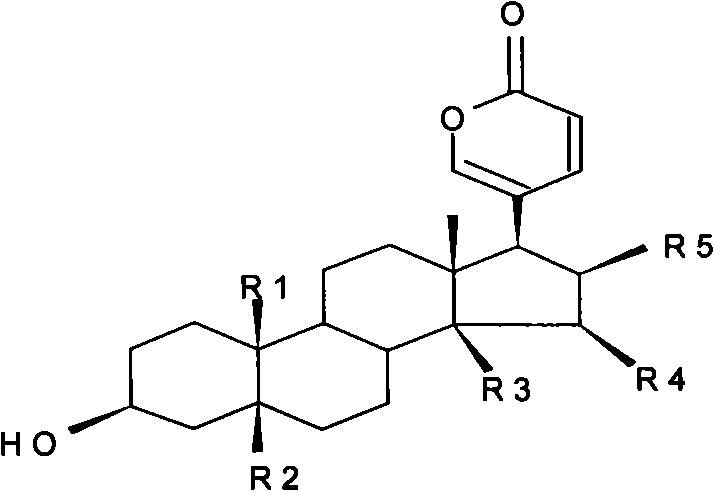
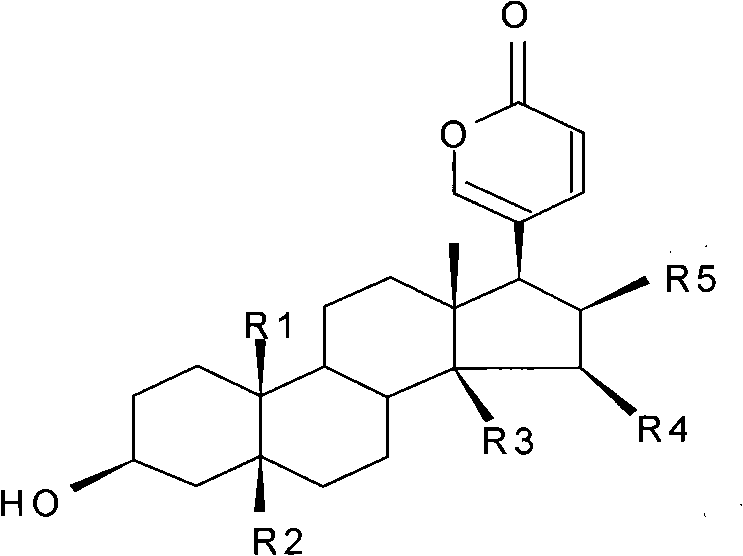
Novel bufadienolide compound as well as preparation method and uses thereof
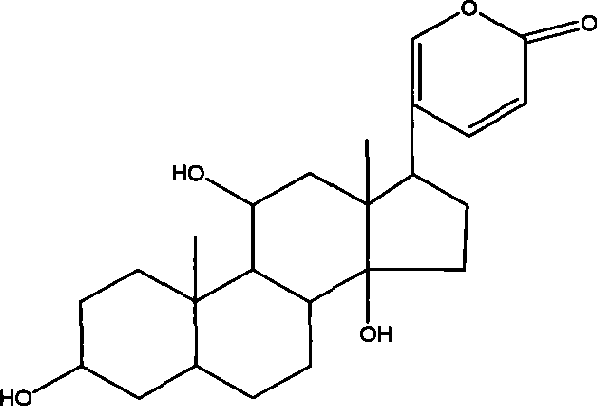
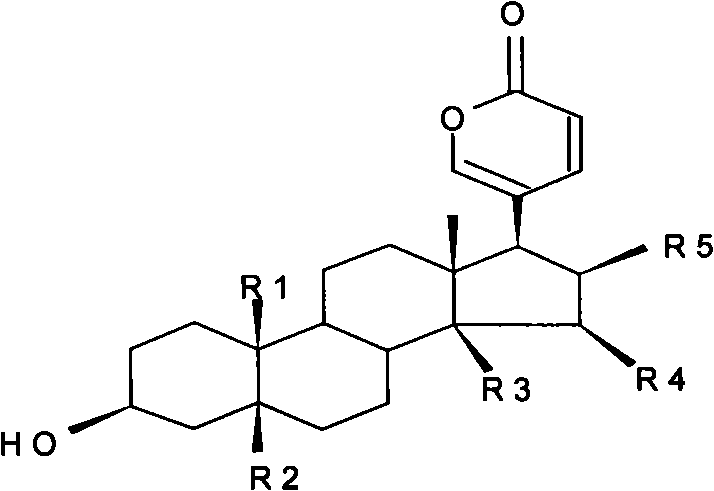
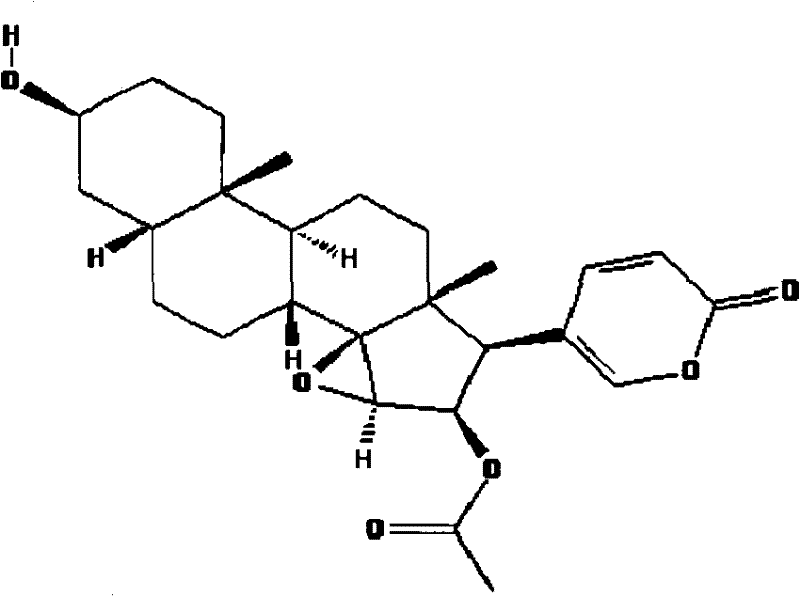
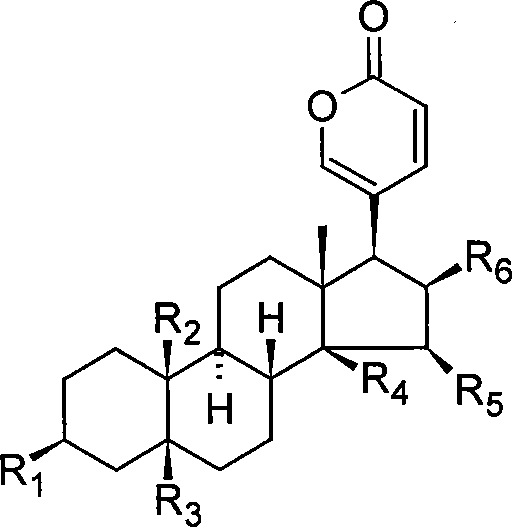
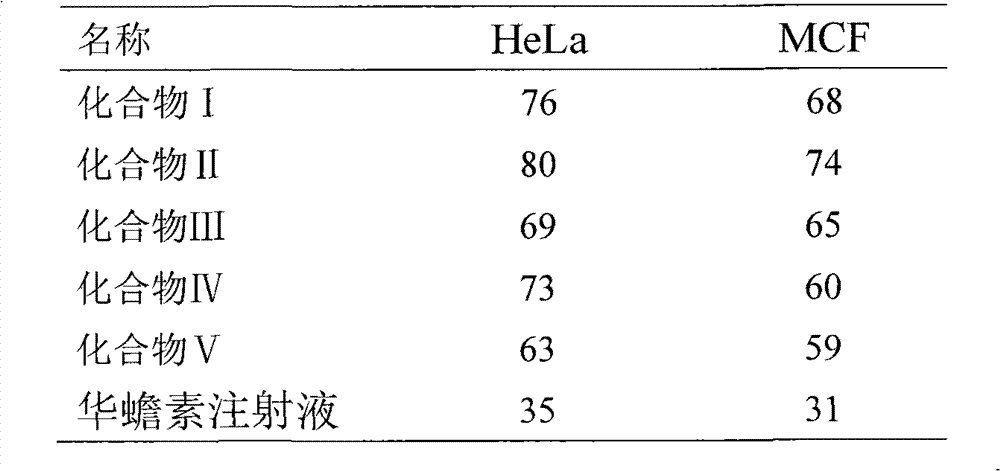
The invention relates to a novel bufadienolides compound and a preparation method and application thereof, and provides a bufadienolides compound having a general formula (I) or pharmaceutical acceptable salts as well as bufadienolides extract or medication composition containing the compound. The compound and the extract have effect for inhibiting tumour cell activity and application to preparation of anti-tumour drugs. The invention also provides the preparation method applicable for industrial production of the compound and the extract.
Owner:SHANDONG LUYE PHARMA CO LTD
Composition with anti-tumor effect and application thereof in preparing medicament for treating tumor
InactiveCN101822832AImprove anti-tumor effectReduce cardiotoxicityAmphibian material medical ingredientsOrganic active ingredientsAntiarrhythmic effectToad Venom
The invention provides a composition with anti-tumor effect and application thereof. The composition consists of 0.01 to 10 weight parts of pure bufadienolide compound, extract rich in bufadienolide compound, raw toad venom or toad skin and 0.01 to 99.9 weight parts of medicament with anti-arrhythmic effect. The composition with the anti-tumor effect has the advantage that the match among the components is scientific and reasonable; multiple in vitro tumor cytotoxicity and in vivo tumor inhibiting experiments show that the composition provided by the invention has good anti-tumor effect and plays a role in synergy after combination; and cardiotoxic experiments show that the cardiotoxicity of the composition provided by the invention is obviously reduced, and the composition can reduce the cardiotoxicity caused by primary single use of the bufadienolide compound, the toad venom or the toad skin and plays a role in detoxifying. Therefore, the composition is safer and more effective to use, and is expected to develop a new generation anti-cancer medicament.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Bufadienolide derivatives, preparing process thereof, composition comprising the same and the use thereof
InactiveUS20130005696A1Easy to prepareSimple synthesizing routeBiocideOrganic active ingredientsMalignancyMedicinal chemistry
The present invention provides a class of bufadienolide derivatives representing by the following formula I or pharmaceutically acceptable salts thereof, the preparing process thereof, pharmaceutical composition comprising the same and the use thereof. The bufadienolide derivatives have inhibitory activities against human-derived tumor cell lines, and thus can be used as a drug for treating malignancies.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Treatment of refractory cancers using Na+/K+-ATPase inhibitors
InactiveUS20080027010A1Improve stress responseGood tumor growthBiocideOrganic active ingredientsATPaseAglycone
The reagent, pharmaceutical formulation, kit, and methods of the invention provides a new approach to treat refractory cancers using Na+ / K+-ATPase inhibitors, such as cardiac glycosides, including bufadienolides or their corresponding aglycones (e.g., proscillaridin, scillaren, and scillarenin, etc.), especially in oral formulations and / or solid dosage forms containing more than 1 mg of active ingredients.
Owner:BIONAUT PHARMA
Toadpoison ligand extract as well as preparation method and application thereof
InactiveCN101176739AAmphibian material medical ingredientsAntineoplastic agentsHigh concentrationToad Venom
The invention provides a bufadienolides extraction extracted from the toad skin or the toad venom; wherein, the weight percentage of the bufadienolides (accounted by the cinobufagin) can reach above 55%. The invention adopts the extraction method that: the toad venom, the toad skin or the bufo siccus is extracted by the ethanol; after the recovery of the ethanol, the macroporous resin column on the concentration liquid is eluted by the water and the low concentration alcohol, and is eluted by the high concentration alcohol, then the final product is gained through collecting the eluent and reducing the pressure of the recovery solvent to pass drying or extraction. The invention has an advantage that: the invention also provides the drug composition using the extraction as the active component, the suppressing the tumour cells active function of the extraction and the application in the preparation of the anti-tumor drug.
Owner:SHANDONG LUYE PHARMA CO LTD
Method for detecting bufadienolide components in Liushen pill
InactiveCN106706812AQuality improvementImprove detection efficiencyComponent separationReference sampleColumn temperature
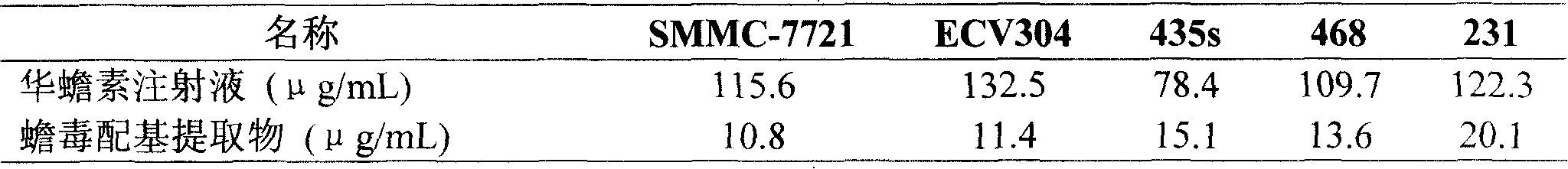
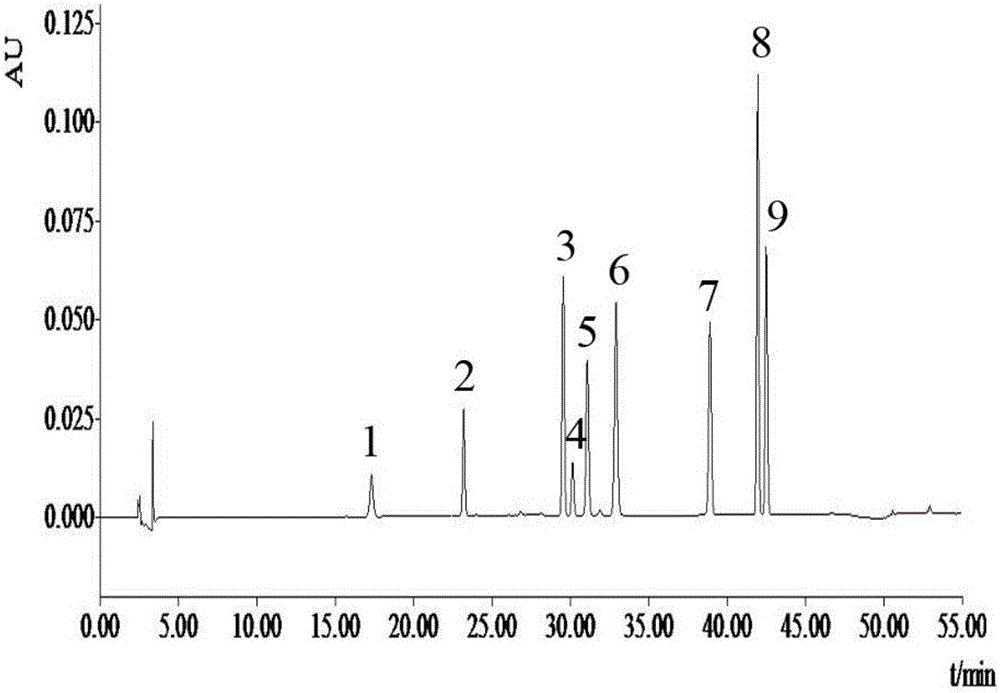
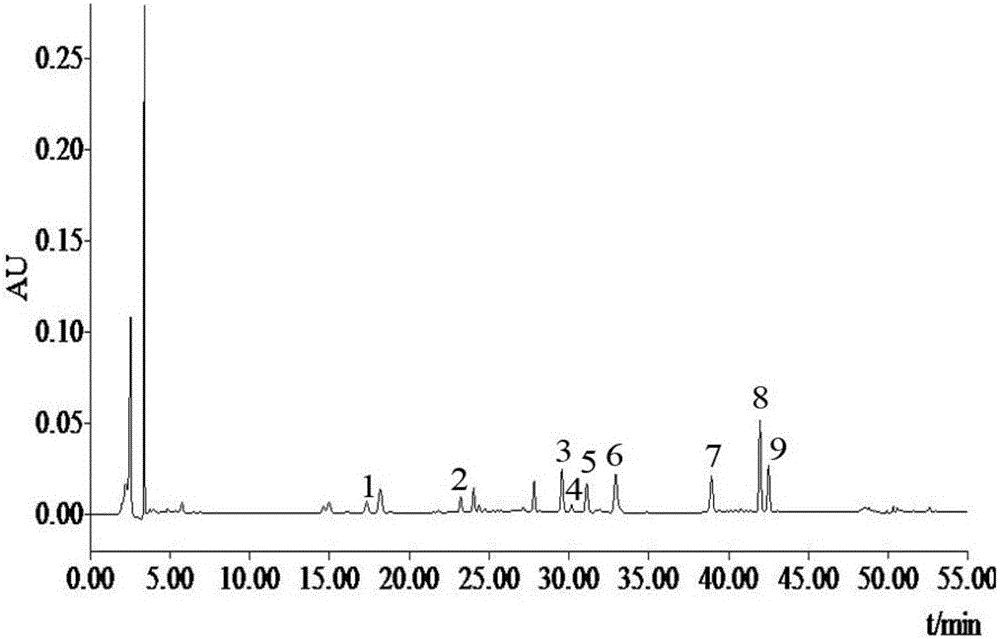
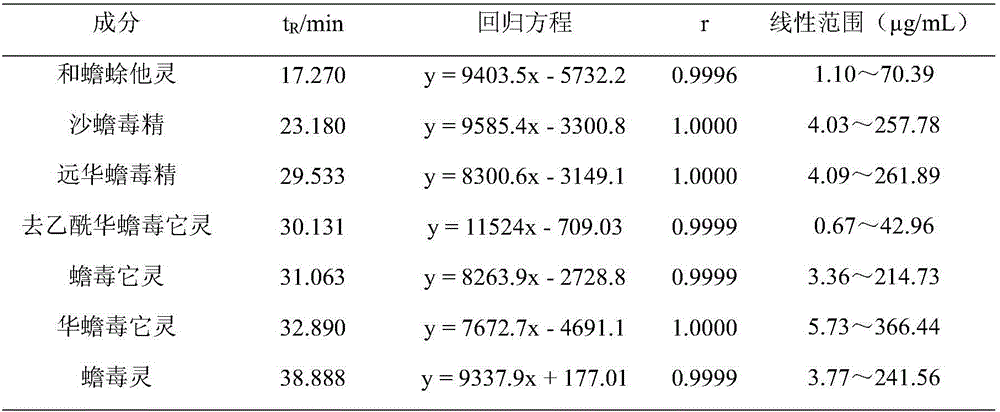
The invention discloses a method for detecting bufadienolide components in a Liushen pill. The method comprises the following steps: preparing a reference sample solution; preparing a test sample solution; and calculating the content through an HPLC assay determination method and a standard curve method. According to the invention, optimal chromatographic conditions such as mobile phase composition, a chromatographic column, an elution program, detection wavelength and column temperature are screened out through a large number of experiments, and the chromatographic conditions are optimized to obtain the optimal detection conditions. The detection method provided by the invention can realize quick, accurate and reliable determination, has high specificity and favorable reproducibility, can simultaneously detect 9 bufadienolide components in the Liushen pill, and can be used for quality control on the Liushen pill, thereby having important significance for drug quality control.
Owner:雷允上药业集团有限公司
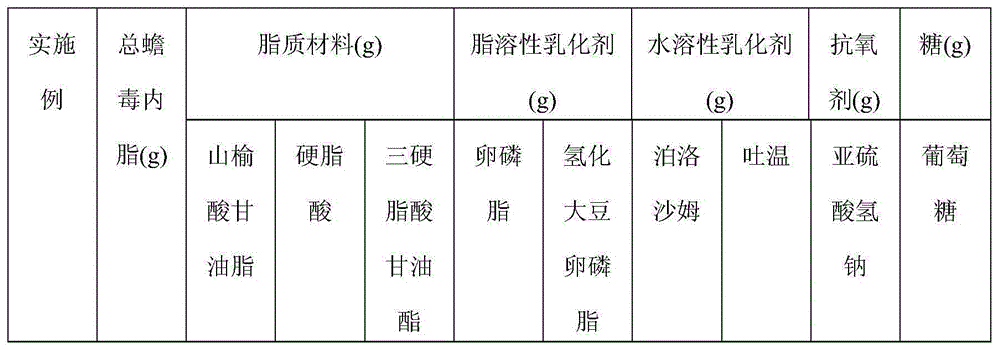
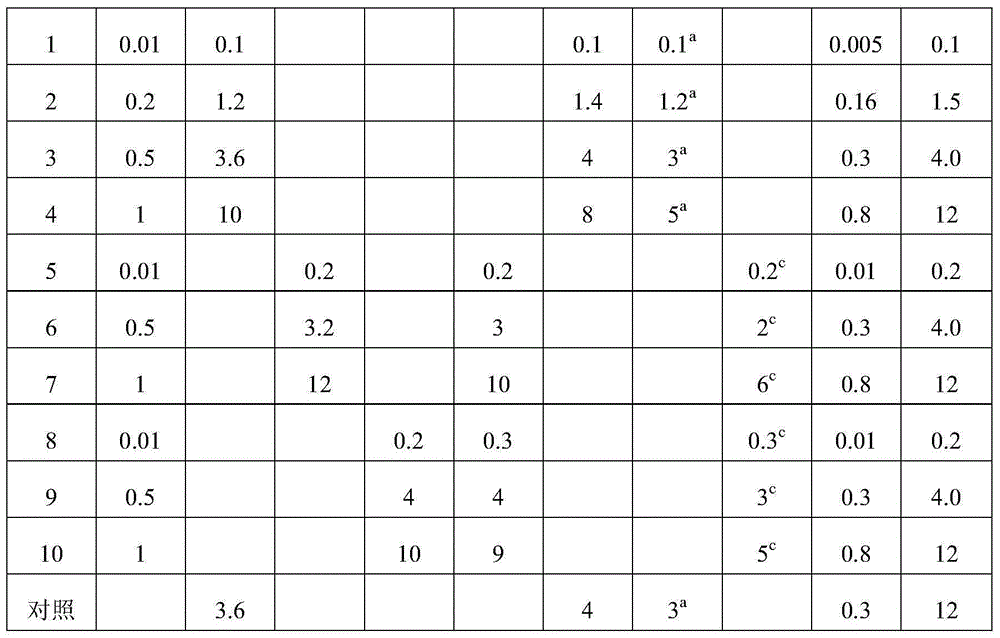
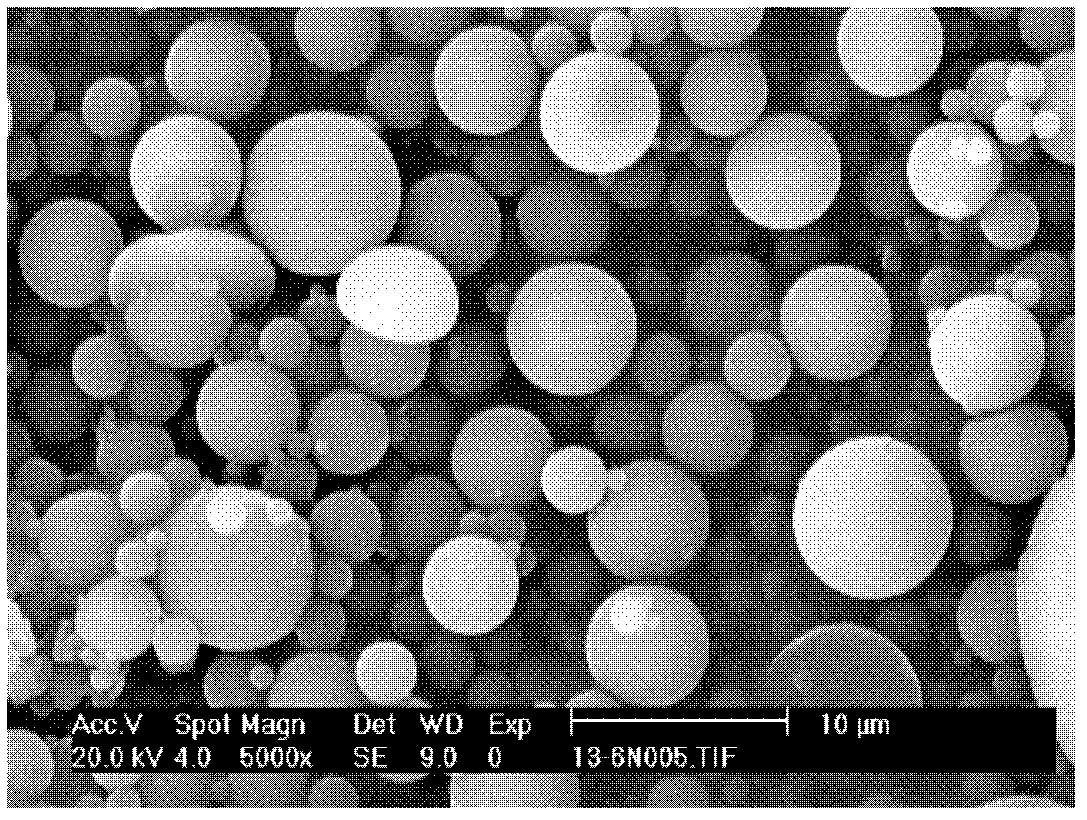
Total bufadienolide solid lipid nanometer particle freeze-dried injection and preparation method thereof
ActiveCN104706622AImprove targetingSmall toxicityAmphibian material medical ingredientsPowder deliverySide effectFreeze-drying
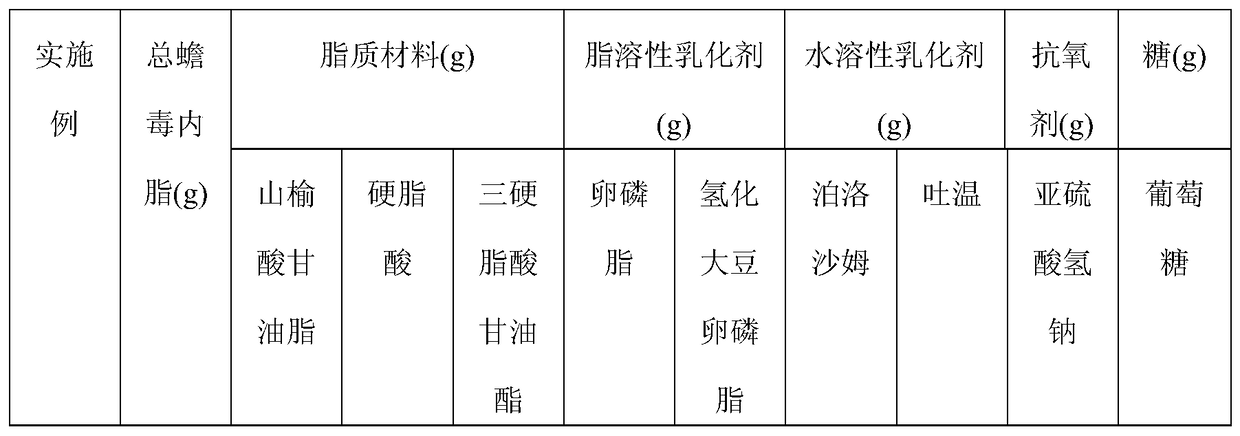
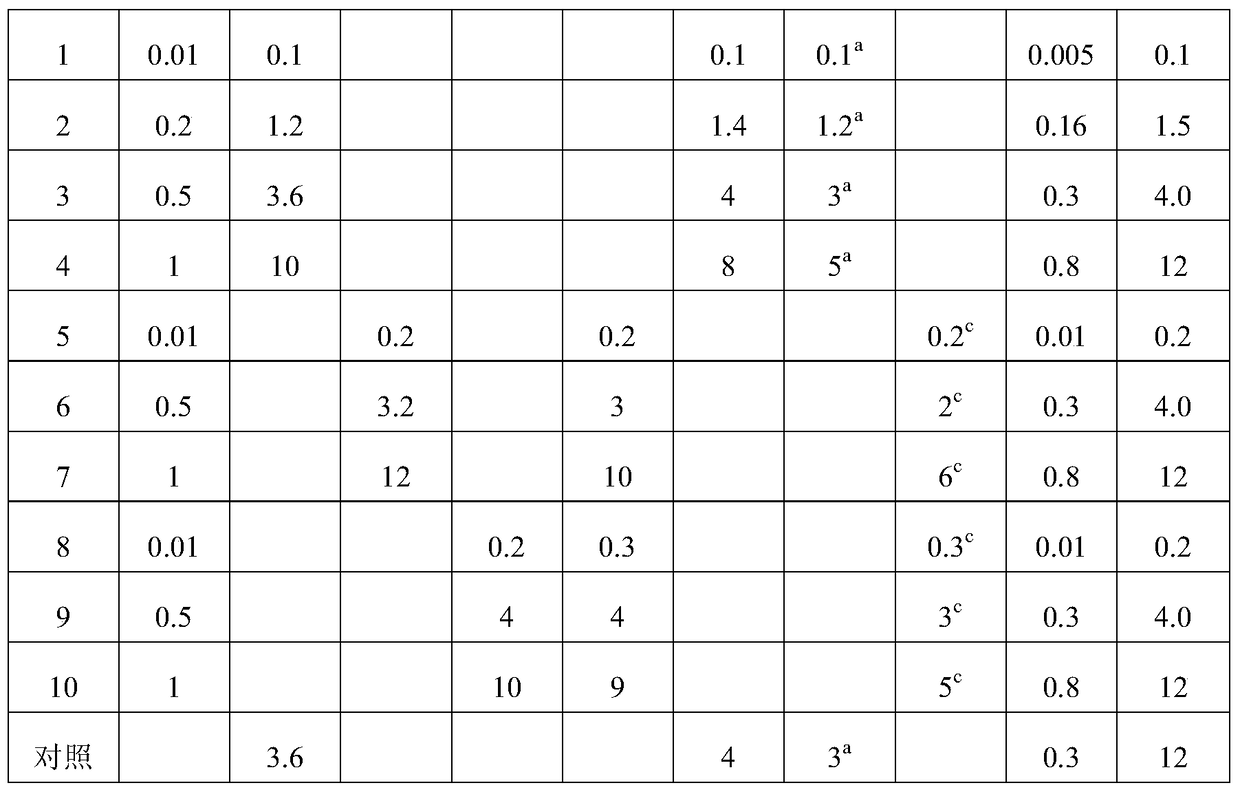
The invention discloses total bufadienolide solid lipid nanometer particle freeze-dried injection and a preparation method thereof and belongs to field of the solid lipid nanometer particle freeze-dried injection and the preparation method thereof. The invention firstly discloses a total bufadienolide solid lipid nanometer particle which comprises the following components: total bufadienolide, solid lipid, a lipid-soluble emulsifying agent, a water-soluble emulsifying agent and water for injection. The total bufadienolide solid lipid nanometer particle is added with a freeze-drying protective agent and then freeze-dried to obtain the total bufadienolide solid lipid nanometer particle freeze-dried injection. After the total bufadienolide solid lipid nanometer particle freeze-dried injection disclosed by the invention is redissolved, the measured particle size of the total bufadienolide solid lipid nanometer particle is 190nm to 250nm, the polydispersity index is 0.18 to 0.3, the encapsulation efficiency is 30% to 80%, the tumor cell targeting property of the total bufadienolide can be effectively improved, and toxic and side effects are alleviated.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
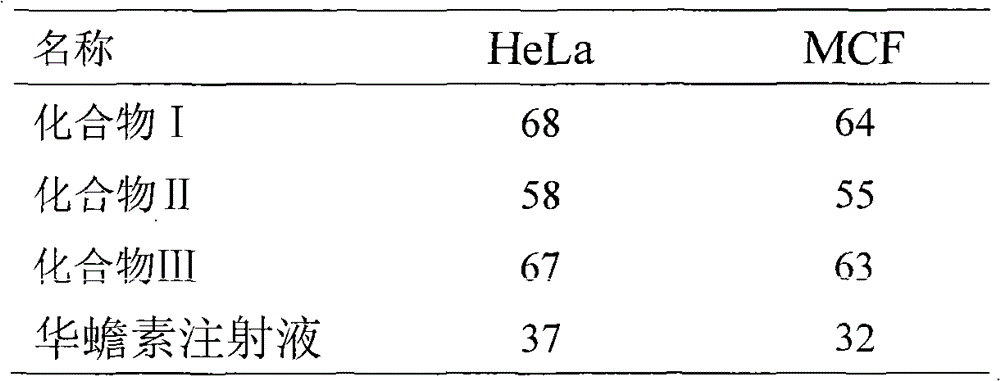
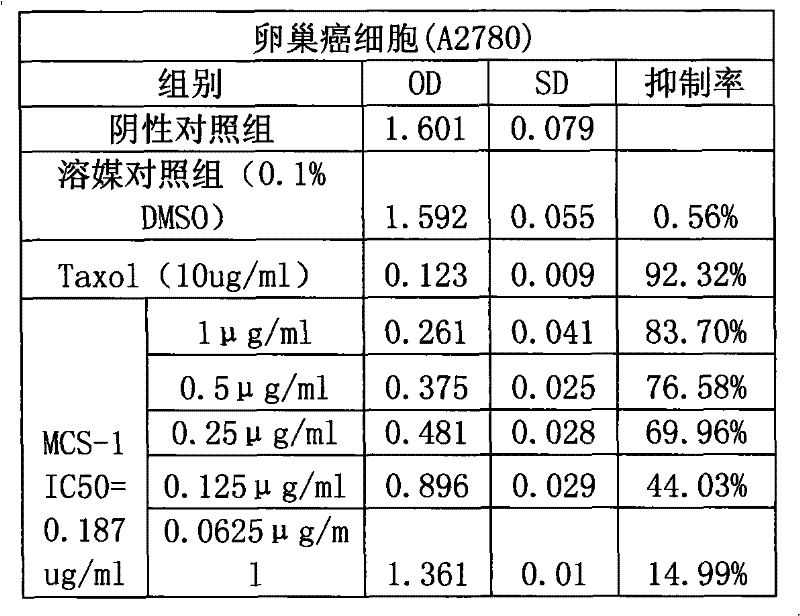
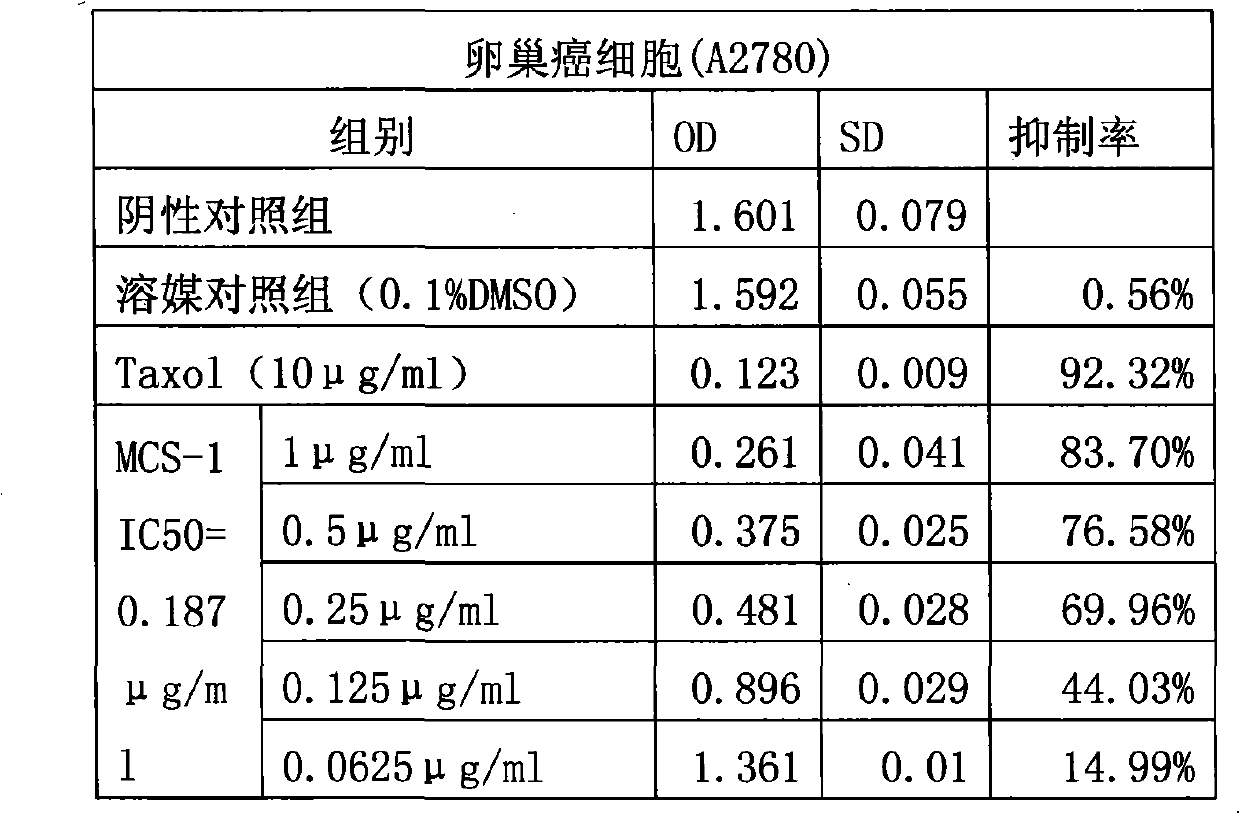
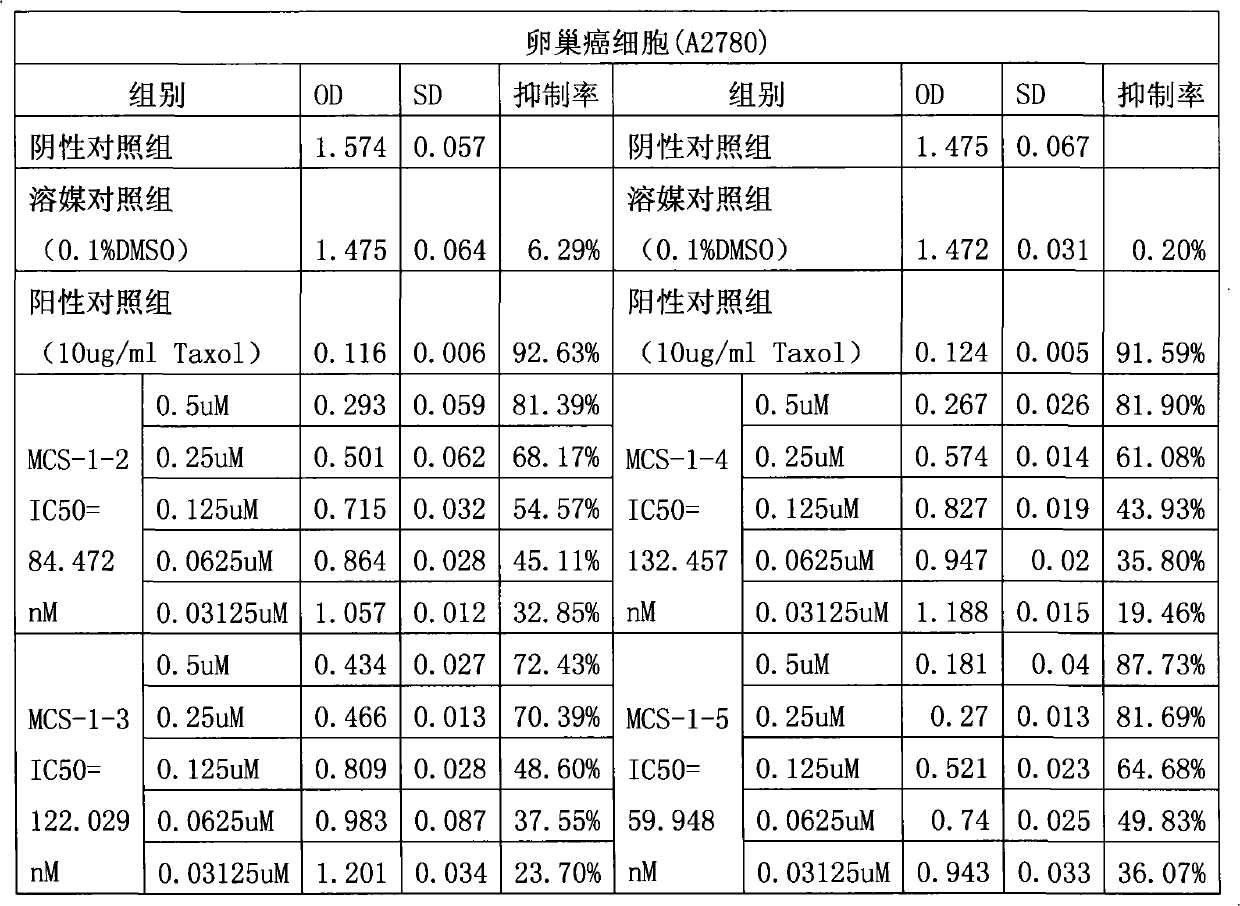
Application of Gamabufotalin and salt thereof in preparing medicament for treating gynaecologic tumor
InactiveCN101485666AActive ingredient clearQuality is easy to controlOrganic active ingredientsSteroidsGynecologic TumorEndometrial cancer
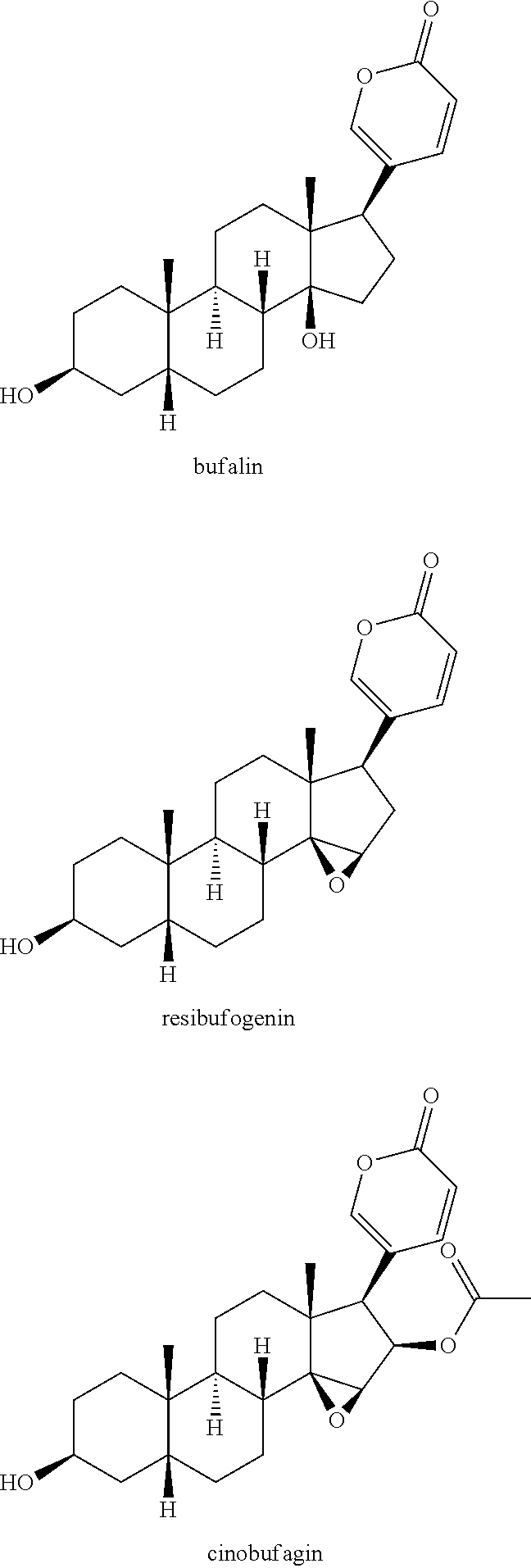
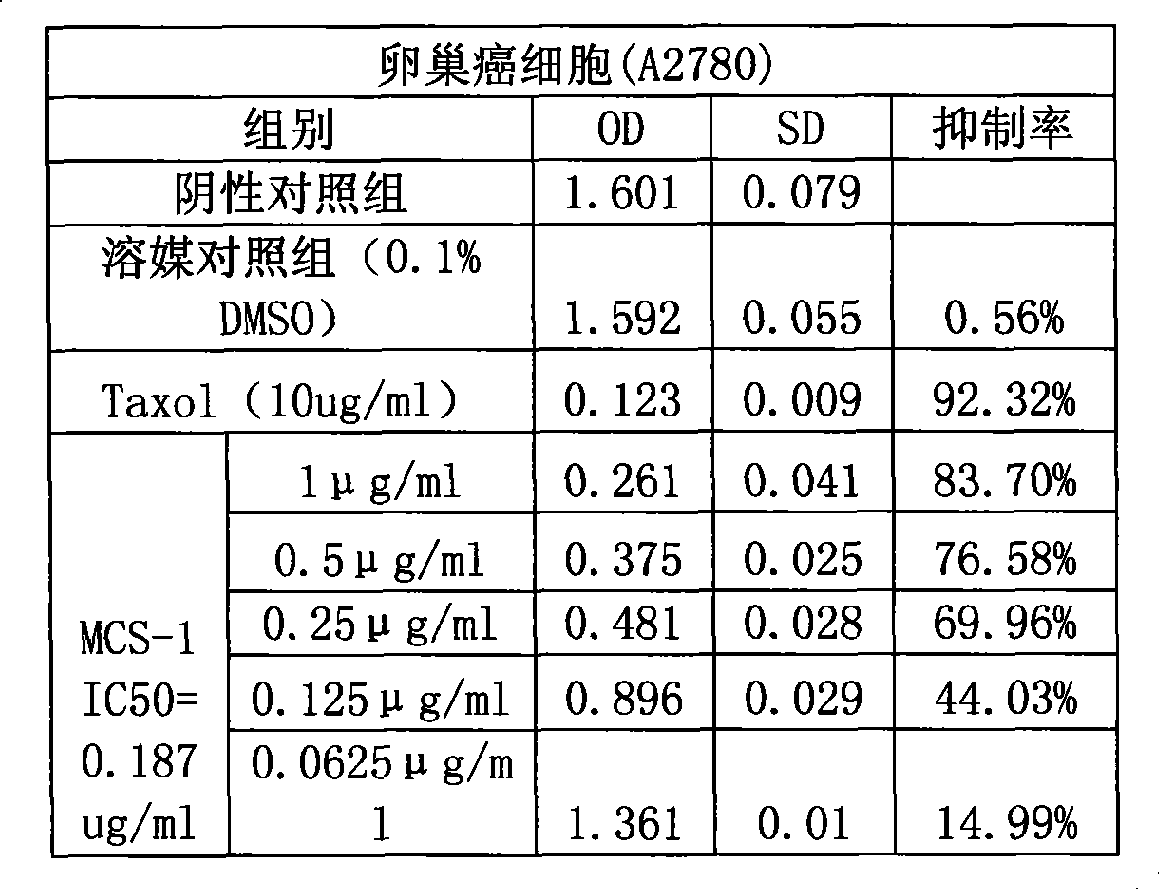
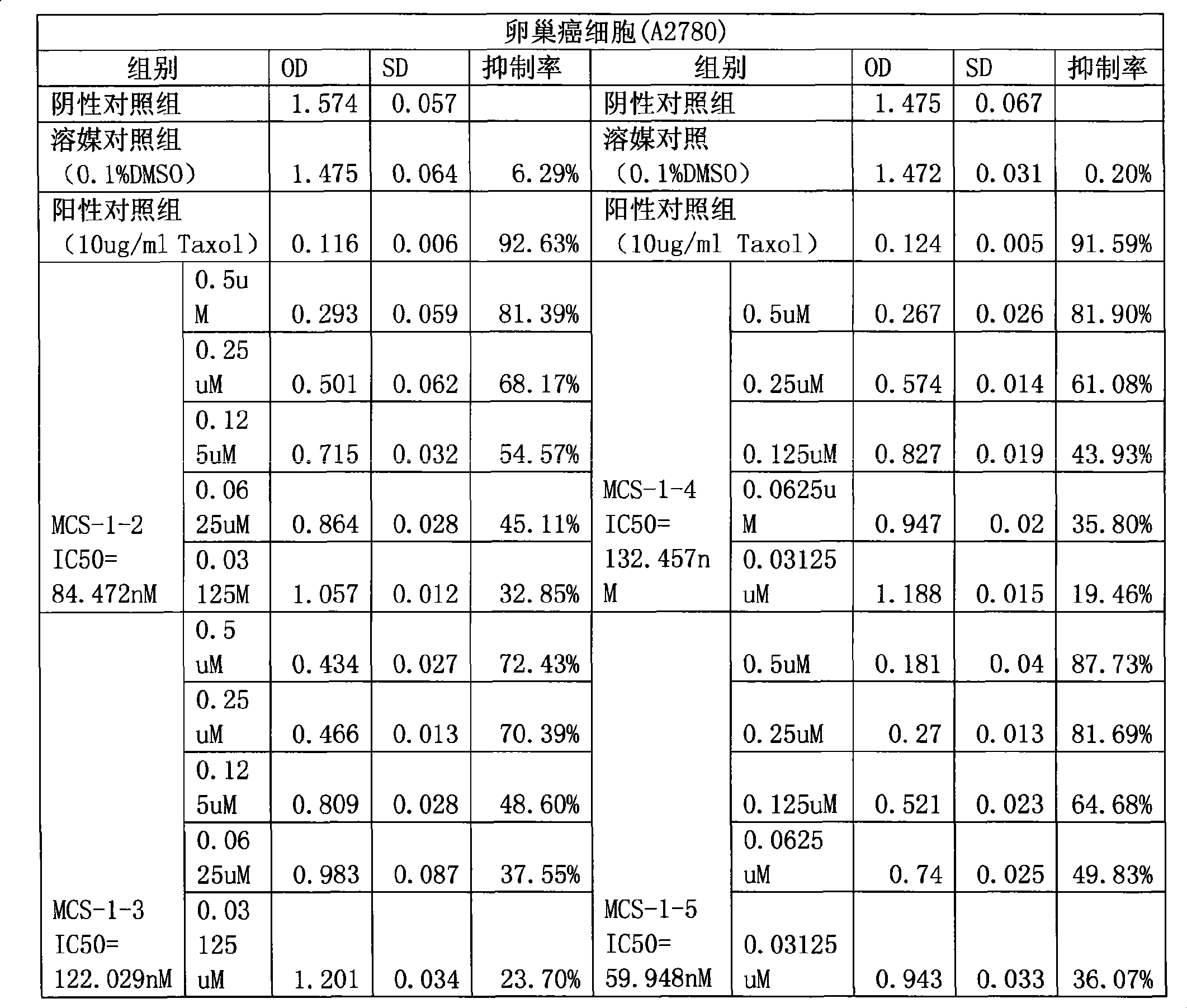
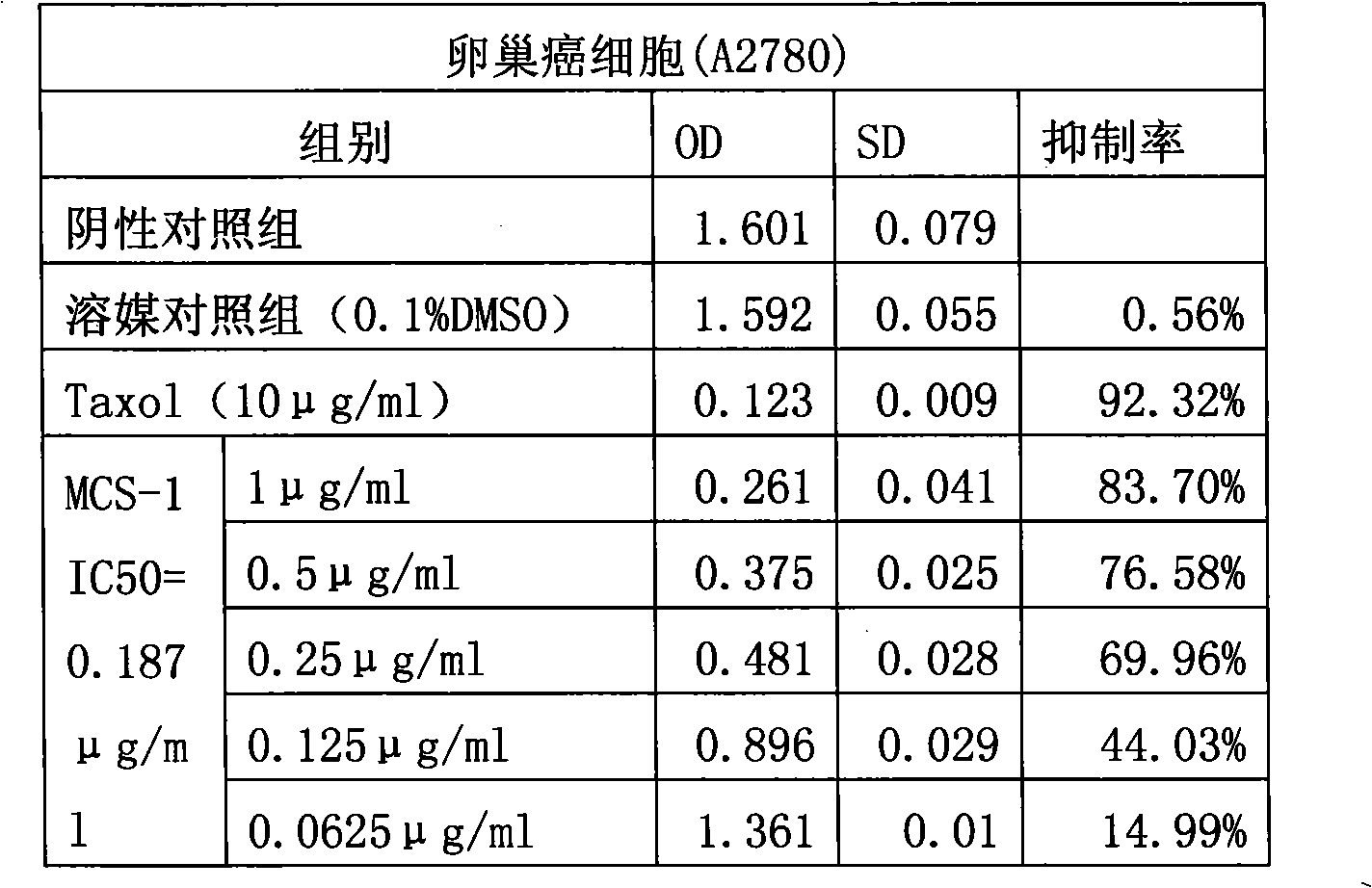
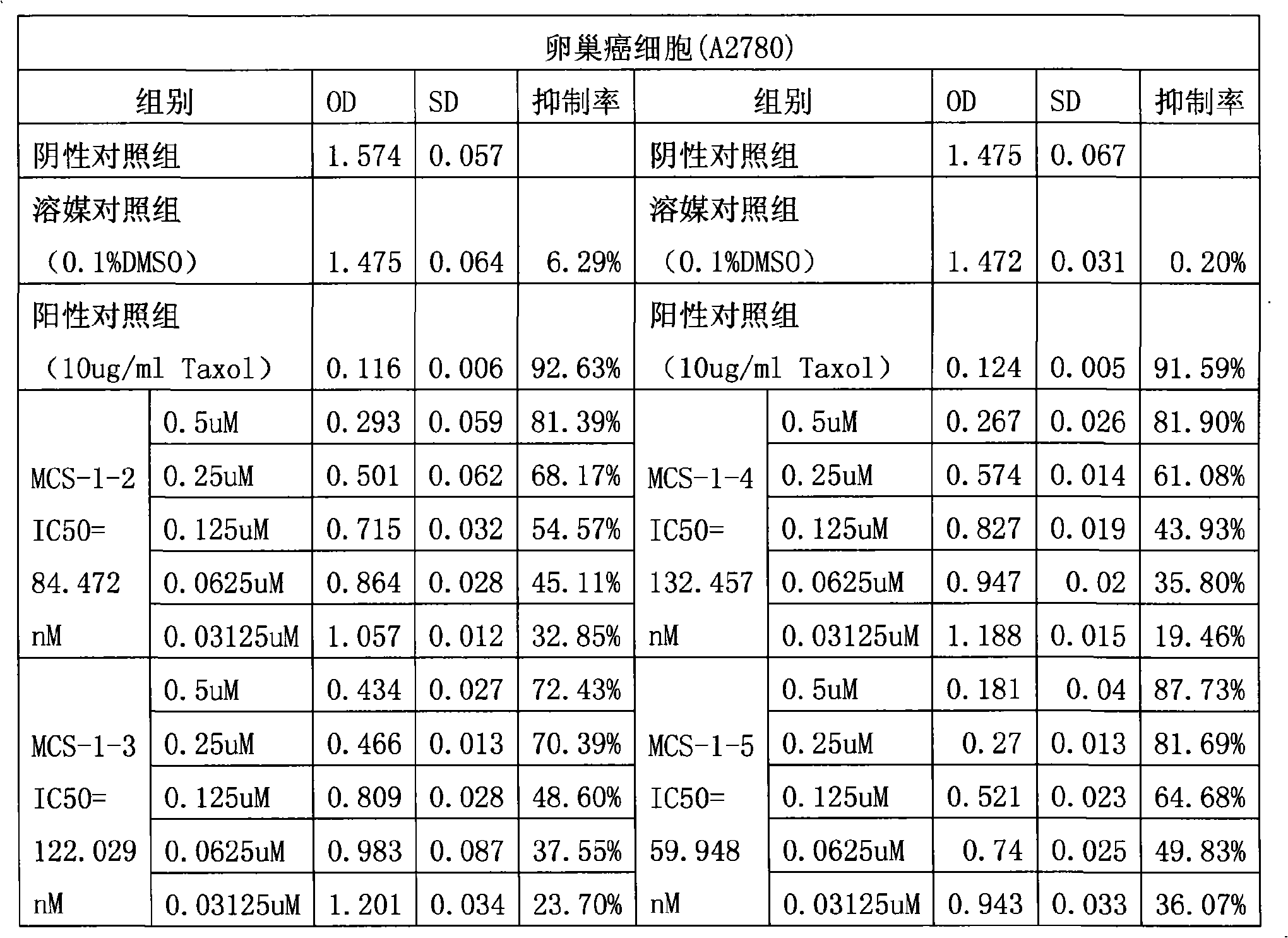
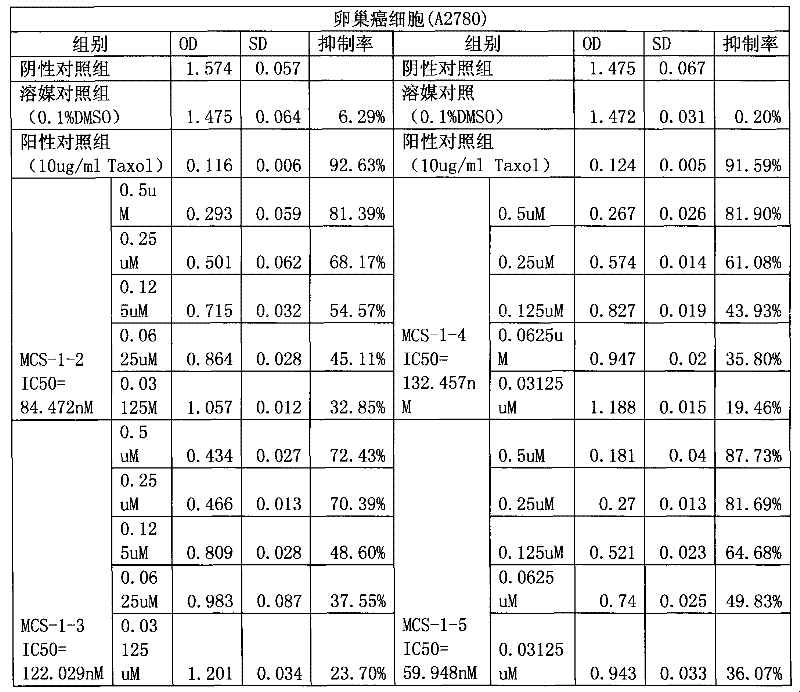
The invention provides application of gamabufotalin and a salt compound thereof in preparation of a medicine for treating gynecological tumor. By comparing in vitro anti-cancer experiments of a bufadienolide extract and monomeric compounds, namely the gamabufotalin, cinobufagin, bufalin and bufotalin as well as hydrochlorides or sulfates of the four compounds to four gynecological tumors, namely human ovarian cancer cells A2780, human ovarian cancer cells SK-OV-3, human cervical carcinoma cells and human endometrial cancer cells, an experimental result shows that the gamabufotalin has stronger activity against gynecological tumor cells than the bufadienolide extract and the monomeric compounds, namely the cinobufagin, the bufalin and the bufotalin, and the hydrochloride or the sulfate of the gamabufotalin has stronger activity against gynecological tumor cells than the hydrochlorides or the sulfates of three compounds, namely the cinobufagin, the bufalin and the bufotalin. The gamabufotalin and the salt compound thereof provided by the invention have the advantages of clear components, controllable quality, strong anti-cancer activity, and small adverse reaction, and are expect to be developed into a novel anti-cancer drug.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Application of venenum bufonis polypeptide as medicine penetration enhancer
ActiveCN110559444AImprove anti-inflammatory and analgesic abilityImprove permeabilityOrganic active ingredientsAntipyreticIn vivoAnalgesics effects
The invention discloses a new application of venenum bufonis polypeptide as medicine penetration enhancers, including bufadienolide and the like. An in vitro Franz diffusion cell and an in vivo transdermal experiment test the penetration enhancement effect of the venenum bufonis polypeptide on the bufadienolide. An LC-MS / MS (Liquid Chromatogram-Mass Spectrometry / Mass Spectrometry) method carries out detection to discover that the penetration of six types of main bufadienolide in cavia procellus skins is increased by 36.99-91.13% after the venenum bufonis polypeptide is added. A von Frey filament stimulation method verifies that the venenum bufonis polypeptide can improve the anti-inflammatory and analgesic capability of the bufadienolide. After the venenum bufonis polypeptide is applied, 2% formalin is injected on the right rear foot of the cavia procellus, a test result indicates that the mechanical withdrawal threshold of the right rear foot of the cavia procellus added with the bufadienolide of the venenum bufonis polypeptide is obviously improved, and the mechanical withdrawal threshold is improved by 2-3 times after the formalin is injected for 30min. The venenum bufonis polypeptide can accelerate the penetration of sterene in the skin of the cavia procellus so as to enhance the anti-inflammatory and analgesic effect of the venenum bufonis polypeptide.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Selective separation and purification method of bufadienolide compounds
InactiveCN104774234AAchieve selective separationEfficient preparationSteroidsChromatographic separationPurification methods
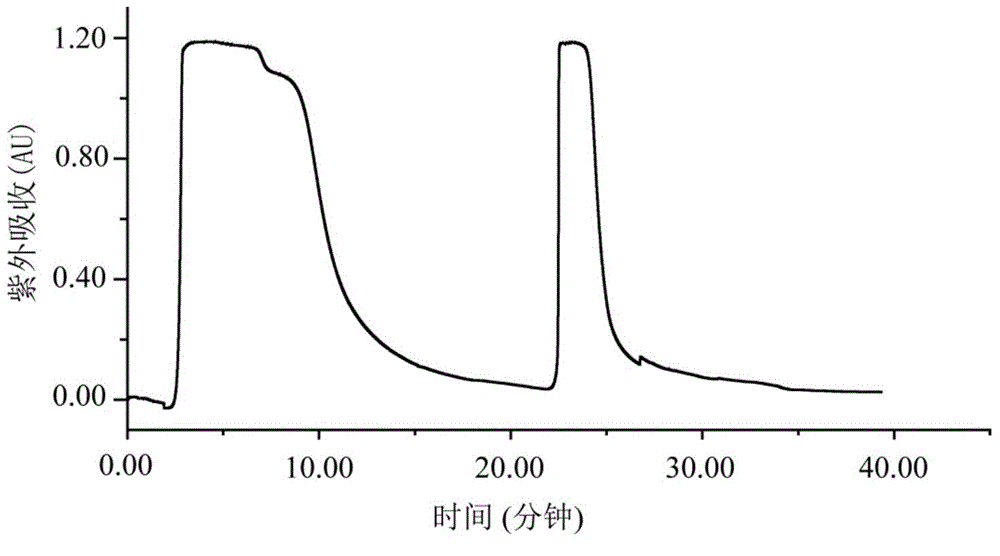
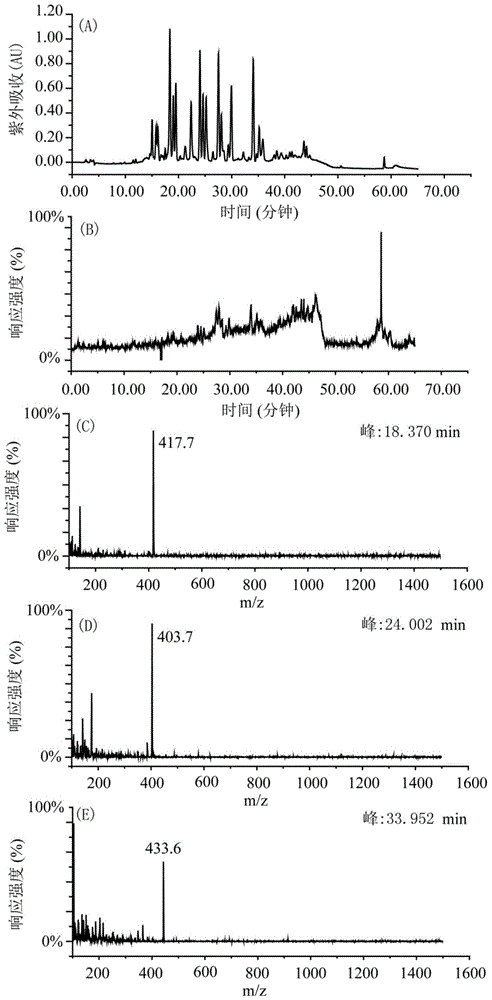
The invention provides a selective separation and purification method of bufadienolide compounds. Positively charged XCharge C18 column is used for developing a novel high-performance liquid chromatographic separation method with high selectivity based on that at a low pH value, ionic properties of amino acid modified bufadienolides are different from ionic properties of bufadienolides which are not modified with amino acid. According to the selective separation and purification method, a mobile phase contains no buffer salt, so that it is convenient for sample preparation postprocessing; a toad skin 98% ethanol extract product is subjected to reversion phase chromatography preparation separation so as to obtain a fraction; and two kinds of compounds, including eight compounds, are obtained via reversed phase chromatography preparation. The selective separation and purification method is capable of realizing high efficiency preparation of amino acid modified bufadienolides and bufadienolides which are not modified with amino acid; and the selective separation and purification method is capable of solving a problem of trailing in separation process of amino acid modified bufadienolides; and excellent peaks are observed, and excellent separation effect is achieved. The selective separation and purification method is used for high efficiency systematic separation of bufadienolides, and is capable of providing substance base and technical support for anticancer active research of bufadienolides and signal-component new drug development.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Use of bufadienolides compound and bufadienolides salinization compound in preparing medicine for treating gynecological tumor
InactiveCN101491531AClear ingredientsLow effective doseOrganic active ingredientsSteroidsGynecologic TumorEndometrial cancer
The invention provides application of a bufadienolide compound and a bufadienolide salt compound in preparing a medicine for treating gynecological tumor. Anti-cancer experiments of four gynecological tumor, namely human ovarian cancer cells (A2780), human ovarian cancer cells (SK-OV-3), human cervical carcinoma cells (SiHa) and human endometrial cancer cells (shikawa) are carried out in vitro through a bufadienolide extract and monomeric compounds, namely cinobufagin, bufalin, bufotalin and gamabufotalin as well as hydrochlorides or sulfates of the four monomeric compounds, and an experimental result shows that the bufadienolide extract, the monomeric compounds, namely the cinobufagin, the bufalin, the bufotalin and the gamabufotalin, and the hydrochlorides or the sulfates of the four monomeric compounds have strong inhibition effect on four gynecological tumor cells. The anti-cancer activity of the bufadienolide compound and the bufadienolide salt compound is stronger than that of a positive drug paclitaxel, and the bufadienolide compound and a bufadienolide salt compound have small adverse reaction and expect to be developed into a novel anti-cancer drug.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
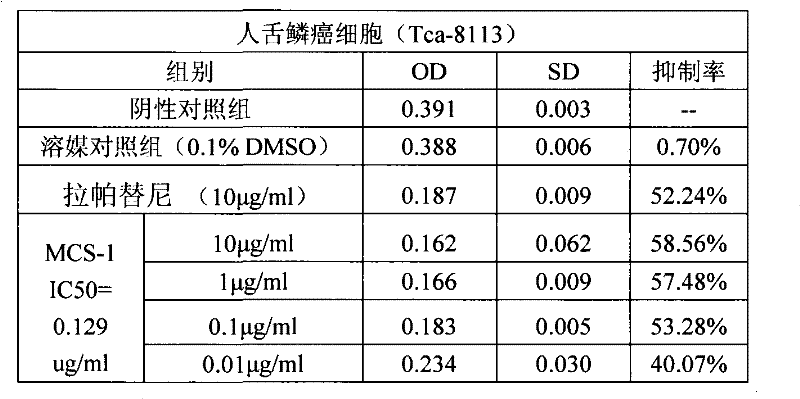
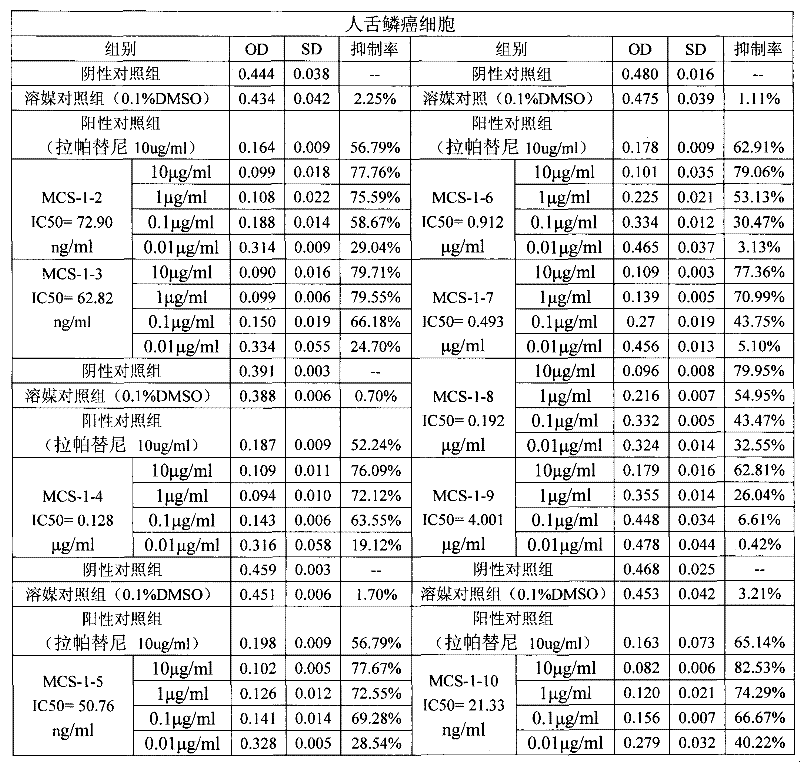
Use of bufadienolide compound in preparing medicines for treating oral mucosal malignant tumors
InactiveCN102688248AActive ingredient clearHigh activityOrganic active ingredientsDigestive systemSquamous cancerOncology drugs
The invention discloses a use of a bufadienolide compound in preparing medicines for treating oral mucosal malignant tumors. Anti-cancer experiments of human tongue squamous cancer cells are carried out in vitro through a bufadienolide extract and monomeric compounds, namely gamabufalin, arenobufagin, telocinobufagin, bufotaline, cinobufagin, desacetylcinobufotalin, heloniogenin, resibufogenin, and cinobufotalin, and experimental results show that the bufadienolide compound has strong inhibition effect on tongue squamous cancer cells and compared with the bufadienolide extract and eight monomeric compounds, namely gamabufalin, arenobufagin, telocinobufagin, bufotaline, desacetylcinobufotalin, heloniogenin, resibufogenin, and cinobufotalin, cinobufagin has a stronger anti-tumor cell activity. The bufadienolide compound provided by the invention has clear components, controllable quality, strong anti-tumor activity and little adverse reaction and is expected to be developed into a novel anti oral mucosal malignant tumor medicine.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
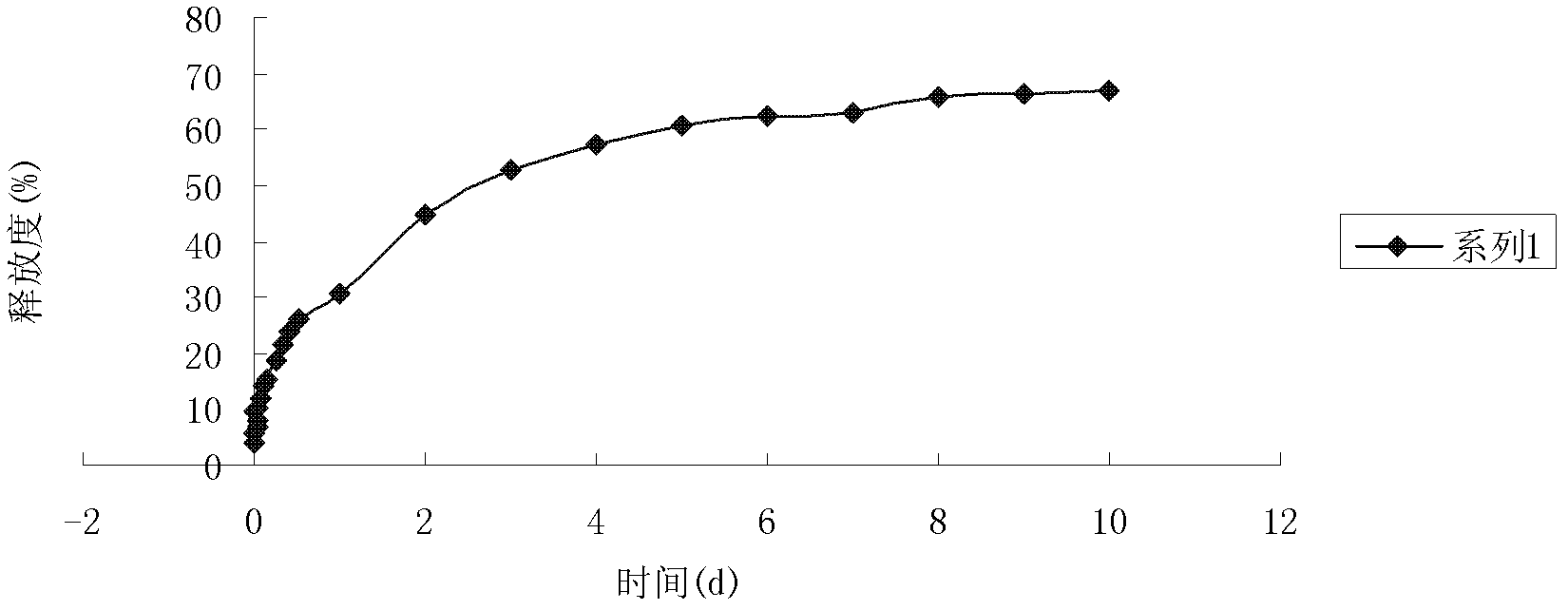
Total bufadienolides solid lipid nanoparticle drug delivery system for injection and preparation method thereof
ActiveCN104997759AEase of industrial productionLow toxicityAmphibian material medical ingredientsDigestive systemSolubilityControl release
The invention belongs to the field of pharmaceutical preparation, and relates to a total bufadienolides solid lipid nanoparticle drug delivery system for injection and a preparation method thereof. Total bufadienolides solid lipid nanoparticles are composed of the components in parts by weight: 0.1-2 parts of total bufadienolides with therapeutically effective amount, 0.5-20 parts of a lipid material, 0.5-15 parts of a lipid soluble emulsifier, 0.5-10 parts of a water soluble emulsifier, 0.002-2.5 part of an antioxidant, 0.1-25 parts of sugar and a proper amount of water for injection. The total bufadienolides solid lipid nanoparticles for injection allow the total bufadienolides to be wrapped in a lipoid nucleus, increase the solubility of the total bufadienolides, reduce the irritation, avoid degradation or leakage of the drug, can achieve targeting effect, controlled release and other effects, and have the advantages of stable physical and chemical properties and the like.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Slow-release microsphere containing venenum bufonis lipoclastic substances as well as preparation method and application thereof
InactiveCN102526111AIncrease doseHigh encapsulation efficiencyAmphibian material medical ingredientsOrganic active ingredientsIrritationPack material
The invention discloses a slow-release microsphere containing venenum bufonis lipoclastic substances as well as a preparation method and application of the slow-release microsphere. The slow-release microsphere is prepared from 0.5 to 30.6 percent of venenum bufonis lipoclastic substances and 69.4 to 99.5 percent of packing materials, wherein the venenum bufonis lipoclastic substances are venenum bufonis extracts using ethanol, ethyl acetate or dichloromethane as solvents, or are bufadienolide monomers obtained from the venenum bufonis lipoclastic substances, and the pakcing materials are preferably selected from polylactic acid or polylactic acid-glycolic acid copolymers. The venenum bufonis lipoclastic substance slow-release microsphere obtained by the invention has the advantages that the medicine loading capability and the encapsulation rate are high, the stability is good, the slow-release effect is obvious, the medicine toxicity can be reduced, the medicine irritation is reduced, the medicine effect time is prolonged, and the medicine administration times are decreased. Various modes such as intravenous injection, intramuscular injection, oral administration and the like are adopted for medicine administration, the slow-release microsphere can be directly used for treating tumor in the medical clinical aspect, or is used for treating various diseases through being matched with other traditional Chinese medicine; and in the veterinary clinical aspect, the slow-release microsphere can be used for treating inflammation or can be used for treating infectious diseases through the combined application with chemotherapy medicine.
Owner:SOUTH CHINA AGRI UNIV
Hellebore petunidin space isomer compound and preparation and application thereof
The invention provides a hellebore petunidin space isomer compound and preparation and application thereof. The hellebore petunidin space isomer compound is prepared from the conventional Chinese medicinal toad skin by using macroporous resin and a two-dimensional preparation chromatographic separation technology. The compound is prepared by mainly comprising the following steps of: cutting the toad skin into pieces; extracting with ethanol; separating a total extract by using the macroporous resin to obtain a toad bufadienolides extract; and performing two-dimensional preparation chromatographic separation and purification to obtain the compound. An in-vitro experiment proves that the compound has a good tumor cell suppression effect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Toadpoison ligand extract as well as preparation method and application thereof
InactiveCN101176739BAmphibian material medical ingredientsOrganic active ingredientsHigh concentrationToad Venom
The invention provides a bufadienolides extraction extracted from the toad skin or the toad venom; wherein, the weight percentage of the bufadienolides (accounted by the cinobufagin) can reach above 55%. The invention adopts the extraction method that: the toad venom, the toad skin or the bufo siccus is extracted by the ethanol; after the recovery of the ethanol, the macroporous resin column on the concentration liquid is eluted by the water and the low concentration alcohol, and is eluted by the high concentration alcohol, then the final product is gained through collecting the eluent and reducing the pressure of the recovery solvent to pass drying or extraction. The invention has an advantage that: the invention also provides the drug composition using the extraction as the active component, the suppressing the tumour cells active function of the extraction and the application in the preparation of the anti-tumor drug.
Owner:SHANDONG LUYE PHARMA CO LTD
Antibodies to bufadienolides prevent inhibition of Na/K ATPase and prolong survival in shock
The present invention relates to compositions and methods of use thereof for treatment of clinical conditions manifested from and / or exacerbated by a decrease or an inhibition of Na / K ATPase activity. The invention relates to composition comprises monoclonal or polyclonal antibodies to bufalin and / or bufalin sulfate, or vaccination against bufalin and / or bufalin sulfate, which prevents or attenuates inhibition of Na / K ATPase activity thereby attenuating the adverse physiological effects of bufalin and / or bufalin sulfate inhibition. The invention also relates to methods of treating hemorrhagic, septic shock, cardiogenic shock, shock resulting from physical trauma, diabetes, mental depression, bipolar disorder and schizophrenia comprising administering a therapeutically effective amount of an bufalin monoclonal or polyclonal antibody.
Owner:SHOCK THERAPEUTICS BIOTECH INC
Novel bufadienolide compound as well as preparation method and uses thereof
The invention relates to a novel bufadienolides compound and a preparation method and application thereof, and provides a bufadienolides compound having a general formula (I) or pharmaceutical acceptable salts as well as bufadienolides extract or medication composition containing the compound. The compound and the extract have effect for inhibiting tumour cell activity and application to preparation of anti-tumour drugs. The invention also provides the preparation method applicable for industrial production of the compound and the extract.
Owner:SHANDONG LUYE PHARMA CO LTD
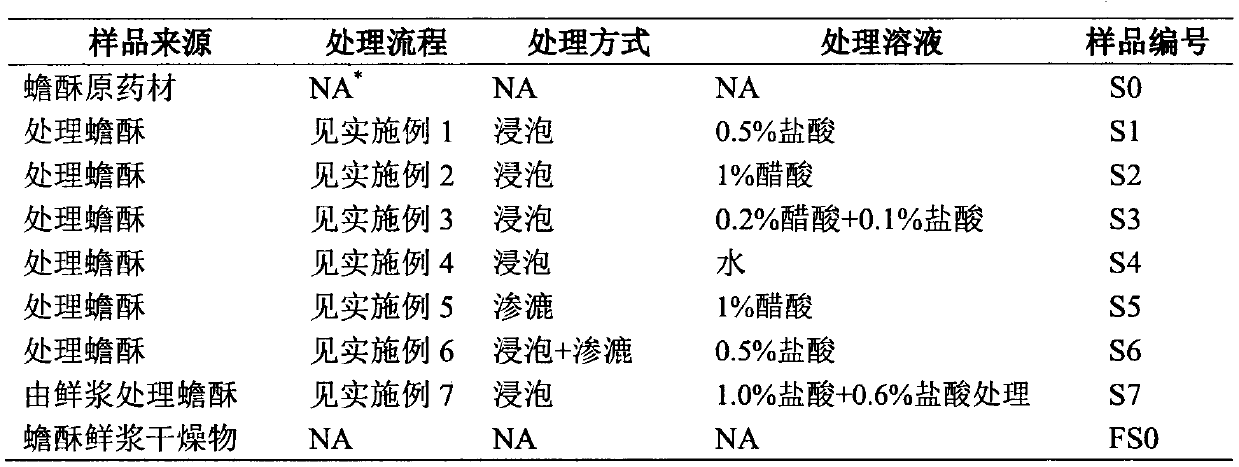
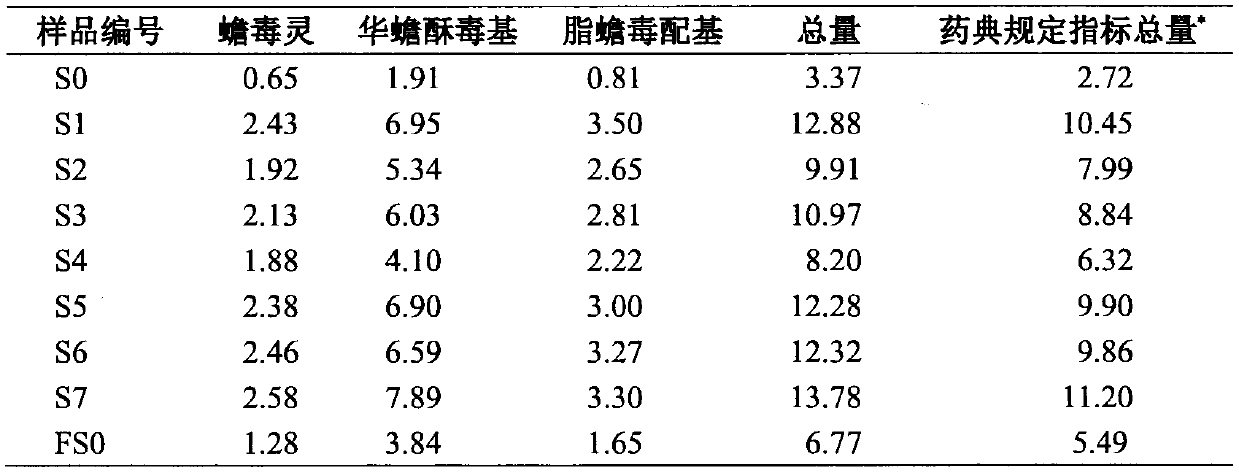
A kind of processing method of traditional Chinese medicine toad venom
ActiveCN104288184BIncrease contentEasy to operateAmphibian material medical ingredientsMedicineAdditive ingredient
The invention discloses a treatment method for processing traditional Chinese medicine venenum bufonis. The treatment method comprises the following steps: adopting water or an acidic aqueous solution to perform extracting treatment on venenum bufonis or a raw material to be processed of venenum bufonis; selecting the solid layer, and drying to obtain the processed venenum bufonis. The treatment method can remarkably improve the contents of the bufadienolide compositions which are the medicinal effective components in venenum bufonis; as numerous venenum bufonis products in the traditional Chinese medicine product market are discrepant to the standard of the contents of the bufadienolide compositions specified by Chinese pharmacopoeia, by adopting the treatment method to process venenum bufonis, the qualification rate of the index of the contents of the bufadienolide compositions specified by Chinese pharmacopoeia can be greatly improved.
Owner:许翔鸿
Method for detecting bufadienolide component in musk Tongxin dropping pill in rat plasma and application of bufadienolide component
PendingCN114858959AShorten detection timeEasy to operateComponent separationAgainst vector-borne diseasesFluid phaseInternal standard
The invention provides a method for detecting bufadienolide components of musk Tongxin dripping pills in rat plasma and application of the method, and particularly relates to a method for qualitatively and quantitatively detecting arenobufogenin, telocinobufagin, deacetylcinobufagin, bufalin and resibufogenin in the rat plasma by adopting ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS / MS). The method comprises the following specific steps: adding an internal standard substance into intragastric musk heart-dredging dropping pill rat plasma, and carrying out methanol protein precipitation; and the detection is carried out through UPLC-MS / MS. Wherein the detection limit of bufalin is 1.00 ng / mL, and the quantification limit of bufalin is 3.00 ng / mL; the detection limit of arenobufogenin, telocinobufagin and resibufogenin is 2.00 ng / mL, and the quantification limit is 6.00 ng / mL; the detection limit of the deacetylated cinobufagin is 3.00 ng / mL, and the quantification limit of the deacetylated cinobufagin is 9.00 ng / mL. According to the method, detection can be completed within 15 min, quantitative calculation is completed through the retention time and the peak area ratio of an MRM chromatogram in combination with a standard curve, operation is more convenient, interference of endogenous substances in plasma is reduced, and the method can be used for pharmacokinetic research of the musk Tongxin dripping pills.
Owner:INNER MONGOLIA CONBA PHARMA CO LTD
Hellebore petunidin space isomer compound and preparation and application thereof
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Application of Gamabufotalin and salt thereof in preparing medicament for treating gynaecologic tumor
InactiveCN101485666BActive ingredient clearQuality is easy to controlOrganic active ingredientsSteroidsCancer cellGynecologic Tumor
The invention provides application of gamabufotalin and a salt compound thereof in preparation of a medicine for treating gynecological tumor. By comparing in vitro anti-cancer experiments of a bufadienolide extract and monomeric compounds, namely the gamabufotalin, cinobufagin, bufalin and bufotalin as well as hydrochlorides or sulfates of the four compounds to four gynecological tumors, namely human ovarian cancer cells A2780, human ovarian cancer cells SK-OV-3, human cervical carcinoma cells and human endometrial cancer cells, an experimental result shows that the gamabufotalin has stronger activity against gynecological tumor cells than the bufadienolide extract and the monomeric compounds, namely the cinobufagin, the bufalin and the bufotalin, and the hydrochloride or the sulfate of the gamabufotalin has stronger activity against gynecological tumor cells than the hydrochlorides or the sulfates of three compounds, namely the cinobufagin, the bufalin and the bufotalin. The gamabufotalin and the salt compound thereof provided by the invention have the advantages of clear components, controllable quality, strong anti-cancer activity, and small adverse reaction, and are expect tobe developed into a novel anti-cancer drug.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
A kind of total bufolide solid lipid nanoparticle drug delivery system for injection and its preparation method
ActiveCN104997759BLow toxicityImprove toleranceAmphibian material medical ingredientsDigestive systemSolubilityLipid formation
The invention belongs to the field of pharmaceutical preparation, and relates to a total bufadienolides solid lipid nanoparticle drug delivery system for injection and a preparation method thereof. Total bufadienolides solid lipid nanoparticles are composed of the components in parts by weight: 0.1-2 parts of total bufadienolides with therapeutically effective amount, 0.5-20 parts of a lipid material, 0.5-15 parts of a lipid soluble emulsifier, 0.5-10 parts of a water soluble emulsifier, 0.002-2.5 part of an antioxidant, 0.1-25 parts of sugar and a proper amount of water for injection. The total bufadienolides solid lipid nanoparticles for injection allow the total bufadienolides to be wrapped in a lipoid nucleus, increase the solubility of the total bufadienolides, reduce the irritation, avoid degradation or leakage of the drug, can achieve targeting effect, controlled release and other effects, and have the advantages of stable physical and chemical properties and the like.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Arenobufagin space isomer compound and preparation and application thereof
The invention provides an arenobufagin space isomer compound and preparation and application thereof. The arenobufagin space isomer compound is prepared from the conventional Chinese medicinal toad skin by using macroporous resin and a two-dimensional preparation chromatographic separation technology. The compound is prepared by mainly comprising the following steps of: cutting the toad skin intopieces; extracting with ethanol; separating a total extract by using the macroporous resin to obtain a toad bufadienolides extract; and performing two-dimensional preparation chromatographic separation and purification to obtain the compound. An in-vitro experiment proves that the compound has a good tumor cell suppression effect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Use of bufadienolides compound and bufadienolides salinization compound in preparing medicine for treating gynecological tumor
InactiveCN101491531BClear ingredientsLow effective doseOrganic active ingredientsSteroidsGynecologic TumorEndometrial cancer
The invention provides application of a bufadienolide compound and a bufadienolide salt compound in preparing a medicine for treating gynecological tumor. Anti-cancer experiments of four gynecological tumor, namely human ovarian cancer cells (A2780), human ovarian cancer cells (SK-OV-3), human cervical carcinoma cells (SiHa) and human endometrial cancer cells (shikawa) are carried out in vitro through a bufadienolide extract and monomeric compounds, namely cinobufagin, bufalin, bufotalin and gamabufotalin as well as hydrochlorides or sulfates of the four monomeric compounds, and an experimental result shows that the bufadienolide extract, the monomeric compounds, namely the cinobufagin, the bufalin, the bufotalin and the gamabufotalin, and the hydrochlorides or the sulfates of the four monomeric compounds have strong inhibition effect on four gynecological tumor cells. The anti-cancer activity of the bufadienolide compound and the bufadienolide salt compound is stronger than that of apositive drug paclitaxel, and the bufadienolide compound and a bufadienolide salt compound have small adverse reaction and expect to be developed into a novel anti-cancer drug.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Composition with anti-tumor effect and application thereof in preparing medicament for treating tumor
InactiveCN101822832BImprove anti-tumor effectReduce cardiotoxicityAmphibian material medical ingredientsOrganic active ingredientsAntiarrhythmic effectToad Venom
The invention provides a composition with anti-tumor effect and application thereof. The composition consists of 0.01 to 10 weight parts of pure bufadienolide compound, extract rich in bufadienolide compound, raw toad venom or toad skin and 0.01 to 99.9 weight parts of medicament with anti-arrhythmic effect. The composition with the anti-tumor effect has the advantage that the match among the components is scientific and reasonable; multiple in vitro tumor cytotoxicity and in vivo tumor inhibiting experiments show that the composition provided by the invention has good anti-tumor effect and plays a role in synergy after combination; and cardiotoxic experiments show that the cardiotoxicity of the composition provided by the invention is obviously reduced, and the composition can reduce thecardiotoxicity caused by primary single use of the bufadienolide compound, the toad venom or the toad skin and plays a role in detoxifying. Therefore, the composition is safer and more effective to use, and is expected to develop a new generation anti-cancer medicament.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
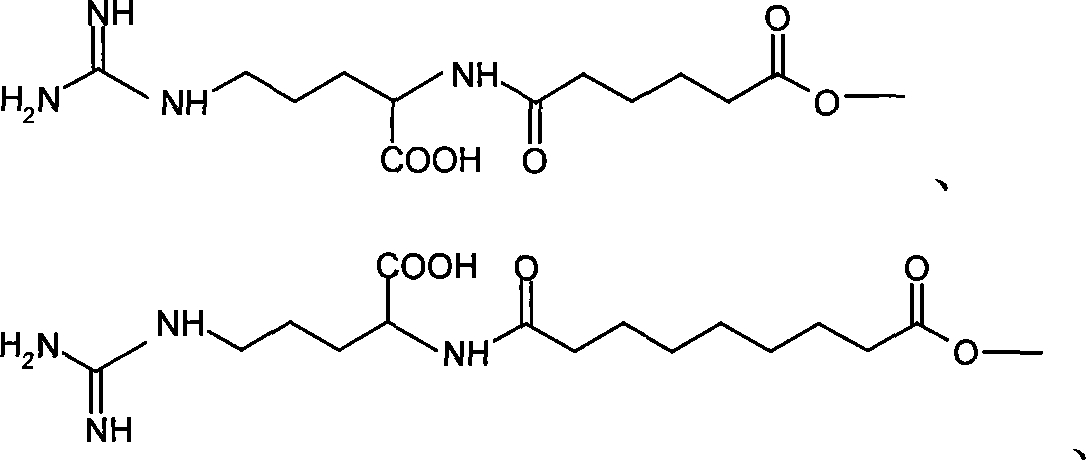
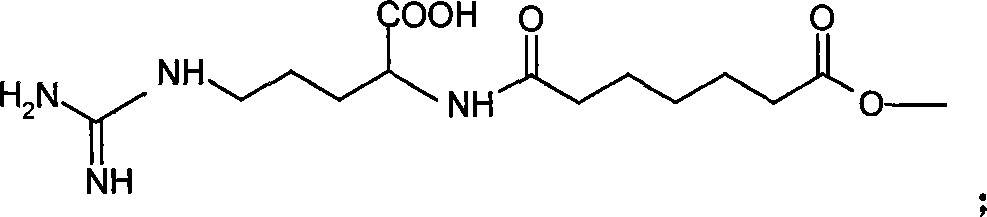
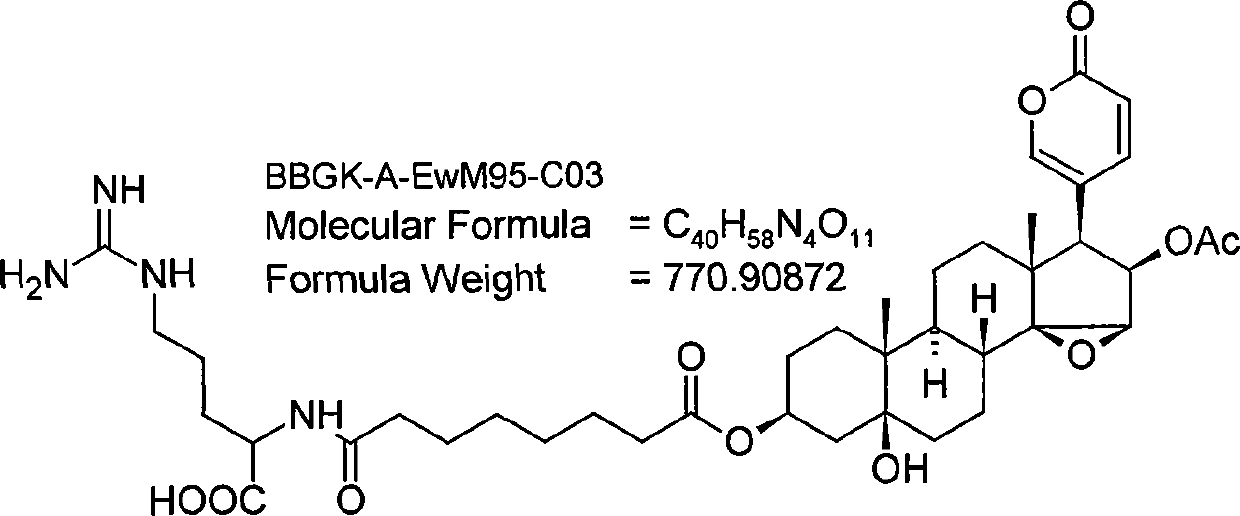
Application of bufadienolide compounds having amino acid chains in preparing anti-tumor drugs
InactiveCN106265693AAmphibian material medical ingredientsOrganic active ingredientsGastric carcinomaColon cancer cell
The invention discloses the influence of an amino acid chain on the anti-tumor activity of toad skin compounds as well as the inhibitory effect of fractions of bufadienolide compounds having the amino acid chains on the growth of colon cancer HT-29 cells and gastric cancer HGC-27 cells. The bufadienolide compounds include a mixture of bufadienolide fractions having and having no amino acid chains as well as a bufadienolide No.1-11 fraction having the amino acid chain, wherein the bufadienolide No.1-11 fraction is separated by virtue of high performance liquid chromatography. In vitro cell experiments indicate that the bufadienolide compounds having the amino acid chains are more significant in inhibitory effect on the growth of colon cancer and gastric cancer than bufadienolide compounds having no the amino acid chains, and meanwhile, some bufadienolide fractions having the amino acid chains take an obvious inhibitory effect on colon cancer cell globules and gastric cancer cell globules; therefore, the bufadienolide fractions having the amino acid chains can be used for preparing medicines for treating the colon cancer and the gastric cancer.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Use of bufadienolide compound in preparing medicines for treating oral mucosal malignant tumors
InactiveCN102688248BActive ingredient clearHigh activityOrganic active ingredientsDigestive systemSquamous cancerArenobufagin
The invention discloses a use of a bufadienolide compound in preparing medicines for treating oral mucosal malignant tumors. Anti-cancer experiments of human tongue squamous cancer cells are carried out in vitro through a bufadienolide extract and monomeric compounds, namely gamabufalin, arenobufagin, telocinobufagin, bufotaline, cinobufagin, desacetylcinobufotalin, heloniogenin, resibufogenin, and cinobufotalin, and experimental results show that the bufadienolide compound has strong inhibition effect on tongue squamous cancer cells and compared with the bufadienolide extract and eight monomeric compounds, namely gamabufalin, arenobufagin, telocinobufagin, bufotaline, desacetylcinobufotalin, heloniogenin, resibufogenin, and cinobufotalin, cinobufagin has a stronger anti-tumor cell activity. The bufadienolide compound provided by the invention has clear components, controllable quality, strong anti-tumor activity and little adverse reaction and is expected to be developed into a novel anti oral mucosal malignant tumor medicine.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
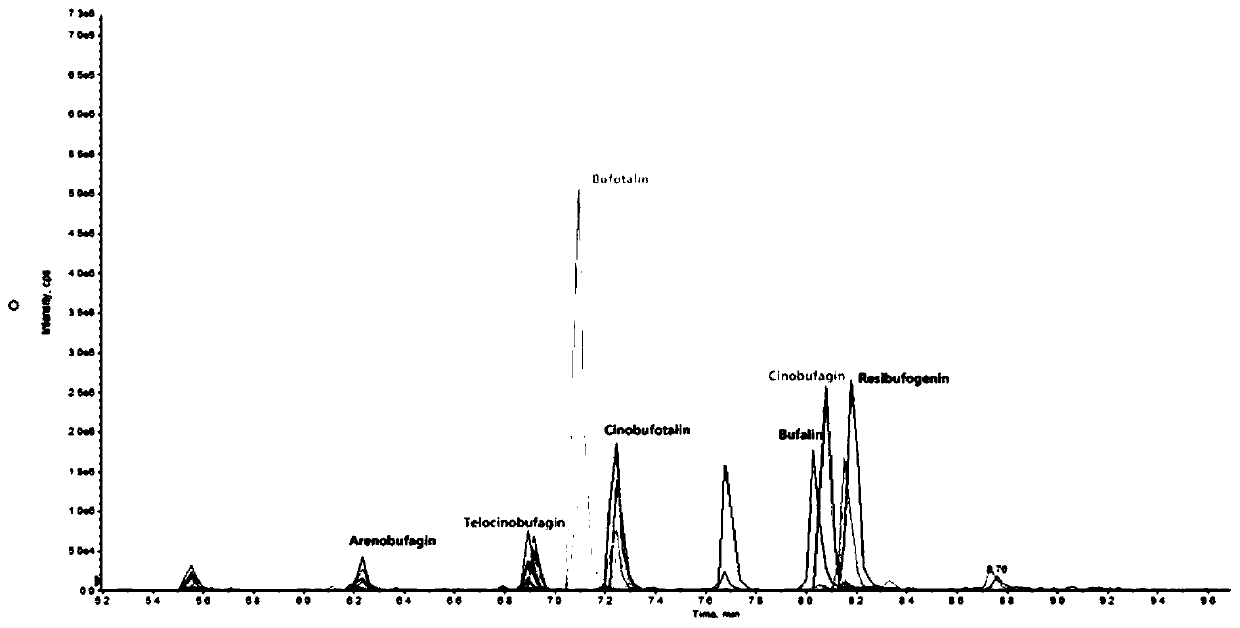
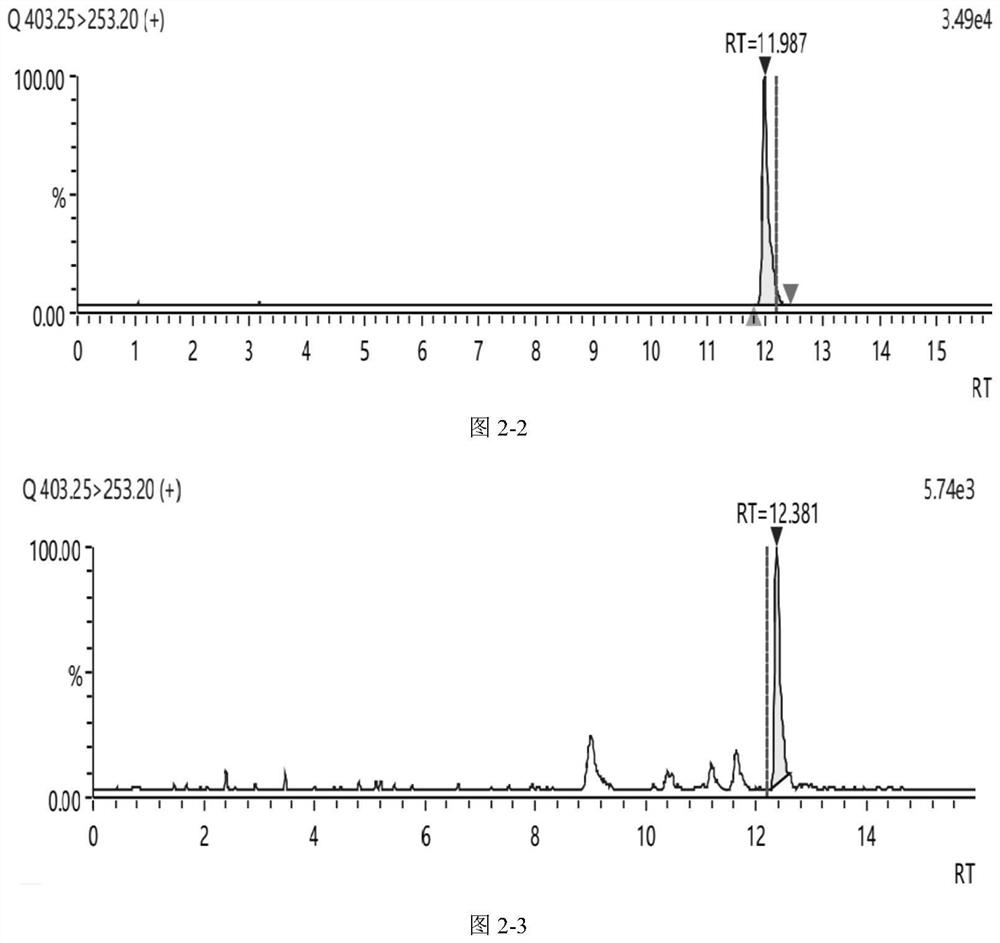
Method for detecting concentration of six bufadienolide components in toad skin-containing medicinal material preparation in blood plasma or tissue
ActiveCN112198251AMeet quantitative requirementsEasy to operateComponent separationMedicinal herbsToad skin
The invention relates to a method for detecting the concentration of six bufadienolide components in a medicinal preparation containing toad skin in blood plasma or tissue. The method comprises the following steps: (1) preparing a stock solution, namely respectively dissolving resibufogenin, bufalin, cinobufagin, cinobufotalin, telocinobufagin and arenobufagin in a (50+ / -5)% acetonitrile aqueous solution to prepare a standard stock solution; dissolving reserpine in a (50+ / -5)% acetonitrile aqueous solution to prepare a reserpine stock solution; (2) preparing a working solution; (3) preparing ato-be-detected sample solution; (4) carrying out liquid chromatography separation on the to-be-detected sample solution obtained in the step (3); and carrying out mass spectrometric analysis on the sample subjected to liquid chromatography separation. In the detection process of the method, the chromatographic peak shape is excellent, a channel of each compound only has a unique target peak, theinterference of other effects such as solvent effects is avoided, and the method is easy to operate, rapid in analysis, high in specificity and high in separation degree.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE
Method for detecting six complex bufadienolide components in medicinal preparation containing toad skin
The invention provides a method for detecting six complex bufadienolide components in a medicinal material preparation containing toad skin. The method adopts high performance liquid chromatography tandem mass spectrometry detection, and comprises the following steps: (1) preparing a stock solution: respectively dissolving resibufogenin, bufalin, cinobufagin, cinobufotalin, telocinobufagin and arenobufagin in an acetonitrile aqueous solution to prepare a standard stock solution; (2) preparing a standard curve solution; (3) preparing a to-be-detected sample solution; (4) carrying out liquid chromatography separation on the to-be-detected sample solution obtained in the step (3); and carrying out mass spectrometric analysis on the sample subjected to liquid chromatography separation. In thedetection process of the method, the chromatographic peak shape is excellent, and a channel of each compound only has a unique target peak, so that interference of other effects such as solvent effects is avoided; and and the method is easy to operate, rapid in analysis, high in specificity and high in separation degree.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com