Slow-release microsphere containing venenum bufonis lipoclastic substances as well as preparation method and application thereof
A technology of toad venom fat-soluble matter and sustained-release microspheres, which is applied in the field of sustained-release preparations of toad venom, can solve problems such as easy demulsification and deterioration, and achieve the effects of prolonging the drug effect time, high encapsulation rate, and reducing the number of administrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The emulsification solvent evaporation method prepares the polylactic acid sustained-release microspheres of the bufo fat-soluble substance, including the following steps:
[0048] After the toad cake is pulverized, it is extracted with dichloromethane, the solvent is distilled off under reduced pressure, and dried in vacuum to obtain a fat-soluble substance of toad venom. Weigh 0.50g of Bufoni fat-soluble matter and 2.85g of polylactic acid with an average molecular weight of 30,000Da, dissolve them in 33mL of dichloromethane, and after they are completely dissolved, add the above solution dropwise to 667mL of 1% (w / v) polyvinyl alcohol (PVA-224) aqueous solution (1% content means that the amount of polyvinyl alcohol is 6.67g), at the same time, under low temperature (ice bath) high-speed (1200r / min) stirring for 8min, the obtained emulsion was stirred at low speed (200r / min) for 6h , centrifuged at 15000r / min for 10min, the precipitate was taken, washed 3 times, and v...
Embodiment 2
[0061] The method of emulsifying solvent evaporation to prepare cinobufaction-based polylactic acid-glycolic acid sustained-release microspheres includes the following steps:
[0062] Weigh 25mg of cinobufaction base and 4.00g of polylactic acid-glycolic acid copolymer with an average molecular weight of 30000Da, dissolve them in 40mL of dichloromethane, and after they are completely dissolved, add the above solution dropwise to 800mL of 1% (w / v) In the aqueous solution of polyvinyl alcohol (PVA-224), at the same time, stir at high speed (1200r / min) at low temperature (ice bath) for 8min, stir the obtained emulsion at low speed (200r / min) for 6h, centrifuge at 15000r / min for 10min, take the precipitate and wash it for 3 3.62 g of cinobufaction-based polylactic-glycolic acid sustained-release microspheres were obtained by vacuum drying.
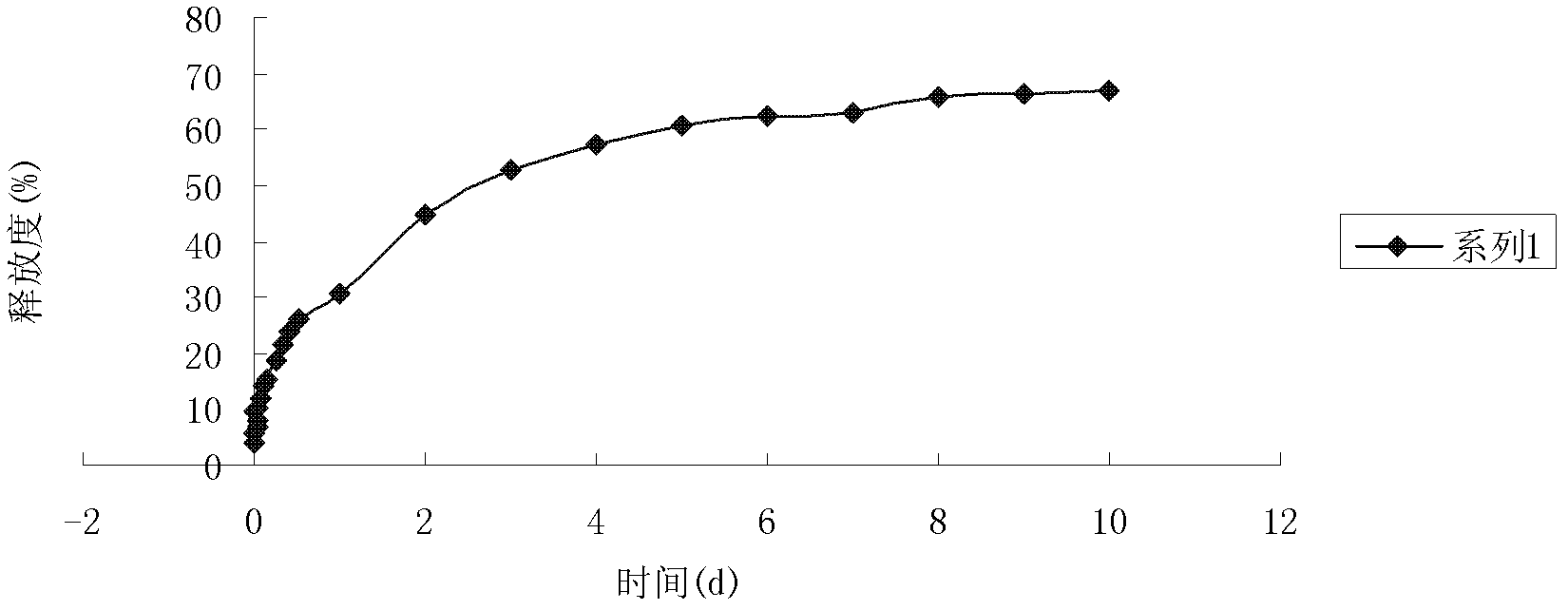
[0063] According to the method for determining the drug loading in Example 1, the content of the cinobufaction base in the slow-release micro...
Embodiment 3
[0065] The spray-drying method prepares the polylactic acid sustained-release microspheres of the bufo fat-soluble substance, comprising the following steps:
[0066] After the toad cake is powdered, it is extracted with ethyl acetate, the solvent is removed by distillation under reduced pressure, and the toad venom is dried in vacuum to obtain a fat soluble substance of toad venom. Weigh 10.00 g of bufo bufo fat solution and 90.00 g of polylactic acid with an average molecular weight of 30,000 Da, dissolve them in 800 mL of ethyl acetate, shake the solution well and spray dry to prepare drug-loaded microspheres. The inlet temperature of the spray dryer was controlled at 50°C, the outlet temperature at 30°C, and the liquid flow rate at 5mL / min. The dry micropowder was collected and weighed 83.50 g.
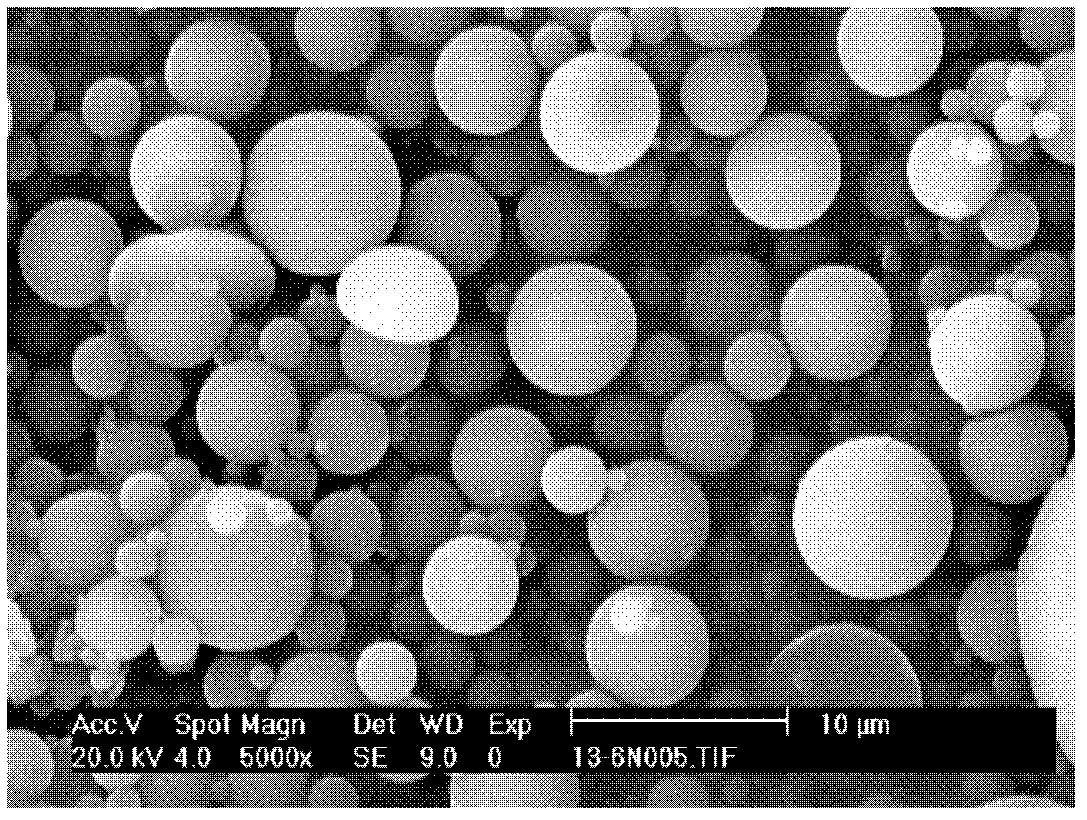
[0067] In this example, the content of the bufo bufo solubles in the sustained-release microspheres was 9.92%. Observing the microspheres with a scanning electron microscope, it...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com