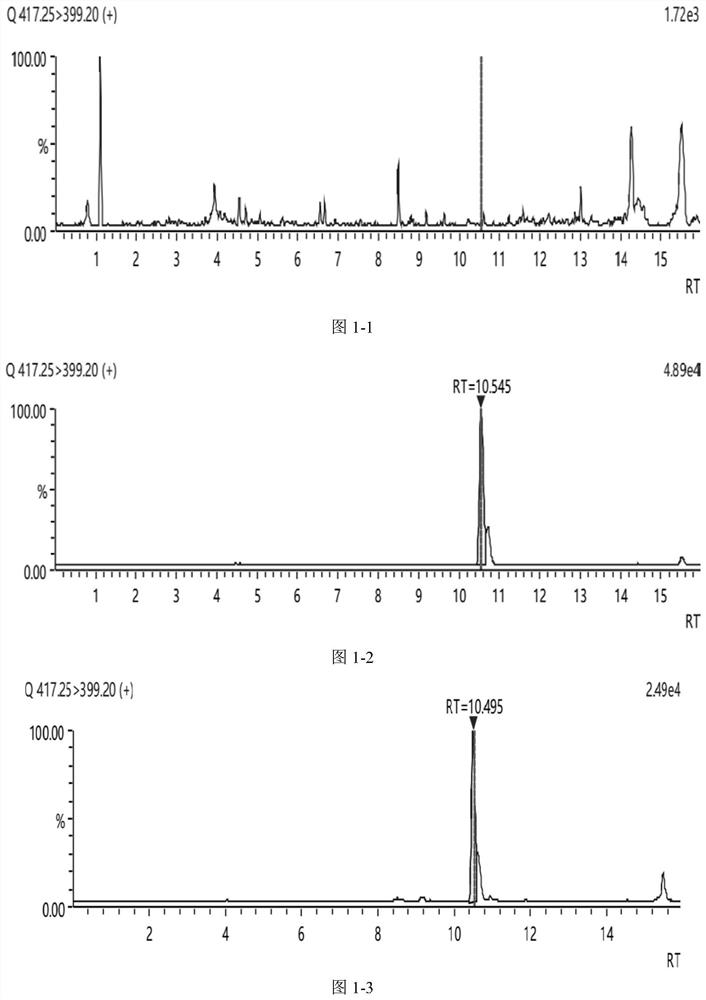
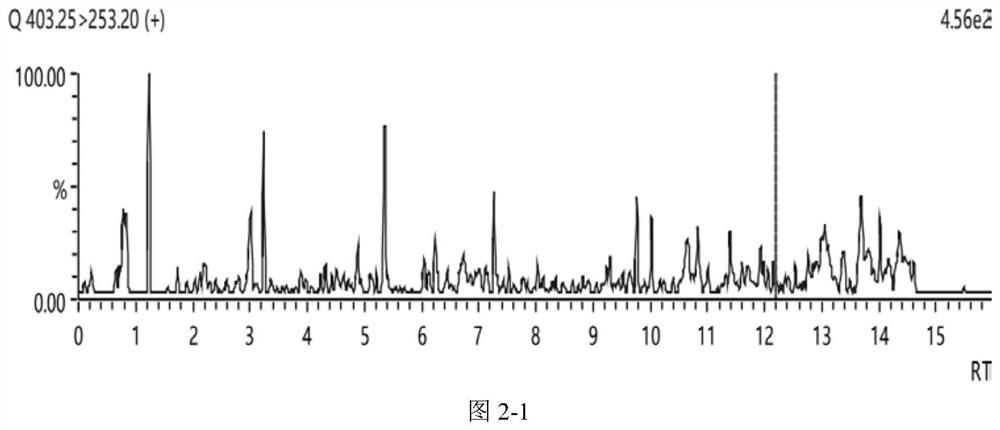
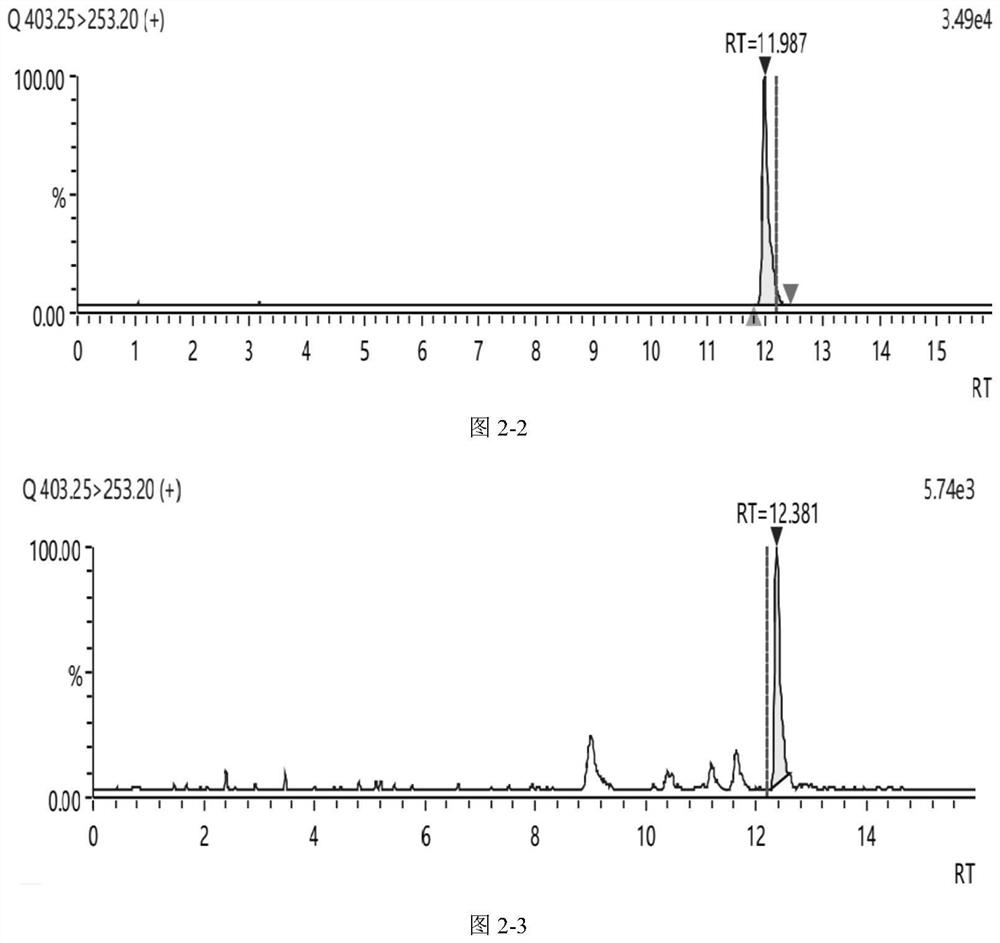
Method for detecting bufadienolide component in musk Tongxin dropping pill in rat plasma and application of bufadienolide component
A technology of bufadienolactone and detection method, which is applied in the direction of measuring devices, material separation, and analysis materials, etc., can solve the problems of complex substrate components, long detection time, and baseline drift of blank serum, and is beneficial to batch detection and detection The effect of shortened time and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Animal feeding, administration and preparation of standard solution
[0044] 1. Animal feeding: SPF grade SD rats, weighing 200g-250g, male. The temperature is 20~26℃, the relative humidity is 40~70%, and the light and dark are alternated for 12h. Free access to water and standard block maintenance diets were provided. Before the test, the animals were acclimated in the experimental animal room for 5 days. Fasting for 12 hours before blood collection, can not help but water.
[0045] 2. Administration: SD rats were weighed, the dosage was calculated, and 6.4 g / kg was administered by oral gavage respectively, and the blank group was given a solvent control.
[0046] 3. Blood collection: add heparin sodium as an anticoagulant in the blood collection tube. The blank blood sample was collected from abdominal aorta, 14 rats, each at least 5 mL, and blood was collected on the same day. Positive blood samples were collected from the ocular venous sinus. The bl...
Embodiment 2
[0048] Example 2: Establishment of detection method 1
[0049] 1. Plasma test sample processing
[0050] Take 200 μL of rat plasma of Shexiang Tongxin Dropping Pills in Example 1, add 5 μL of tinidazole solution with a concentration of 2 μg / mL, mix well, add 1 mL of methanol, vortex for 5 min, and centrifuge at 10,000 r / min at 4°C for 10 min; The supernatant was blown to near dryness with nitrogen at room temperature, reconstituted by adding 300 μL of methanol, vortexed for 5 min, and centrifuged at 10000 r / min at 4°C for 5 min. The supernatant was filtered through a membrane to prepare a plasma test sample.
[0051] 2. Standard curve preparation
[0052] The standard solution of bufadienolide was added to the blank plasma to prepare a series of plasma standard curve samples; the method of step (1) was used to replace the plasma standard curve samples by gavage of the rat plasma of Shexiang Tongxin Dropping Pills to prepare standard curve samples. Curve plasma samples; injec...
Embodiment 3
[0075] Example 3: Establishment of detection method 2
[0076] 1. Plasma test sample processing
[0077] Take 100 μL of rat plasma of Shexiang Tongxin Dropping Pill by gavage in Example 1, add 2.5 μL of tinidazole solution with a concentration of 1 μg / mL, mix well, add 0.3 mL of methanol, vortex for 7 min, and centrifuge at 8000 r / min at 2°C for 5 min; Take the supernatant, blow it to near dryness with nitrogen at room temperature, add 200 μL of methanol to reconstitute, vortex for 5 min, centrifuge at 8000 r / min at 2°C for 5 min, take the supernatant filter membrane, and prepare a plasma test sample.
[0078] 2. Standard curve preparation
[0079] The standard solution of bufadienolide was added to the blank plasma to prepare a series of plasma standard curve samples; the method of step (1) was used to replace the plasma standard curve samples by gavage of the rat plasma of Shexiang Tongxin Dropping Pills to prepare standard curve samples. Curve plasma samples; inject the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com