Selective separation and purification method of bufadienolide compounds
A technology for the separation and purification of bufadienolactones, which is applied in the fields of steroids and organic chemistry, can solve the problems of time-consuming separation and purification methods, complex structures, large quantities, etc., and achieve the effect of efficient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
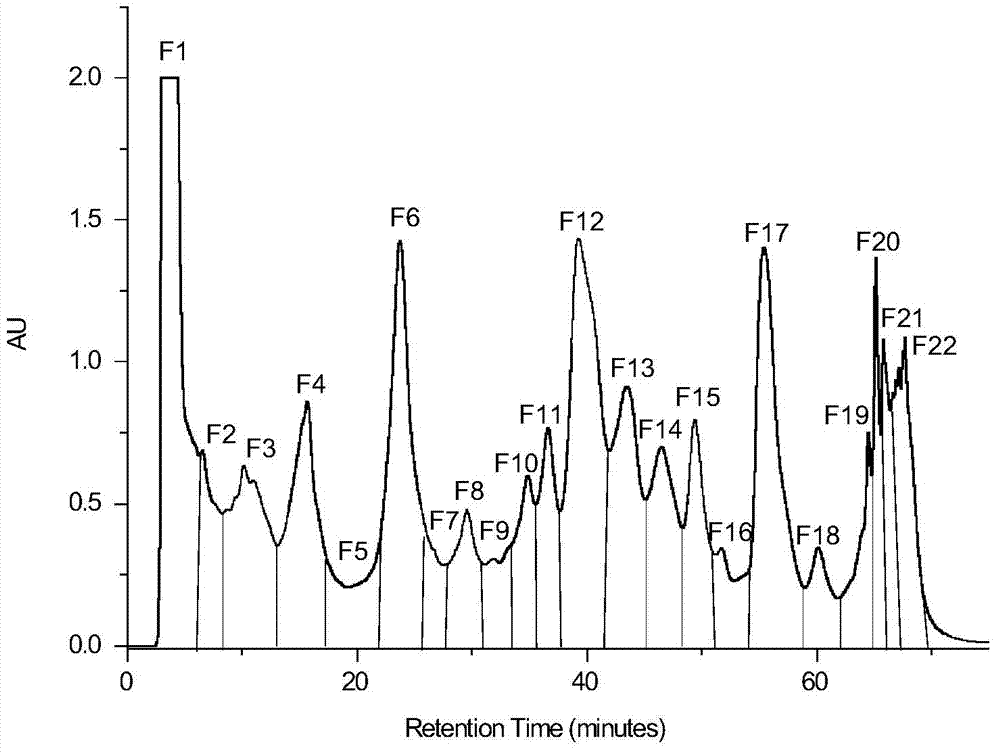
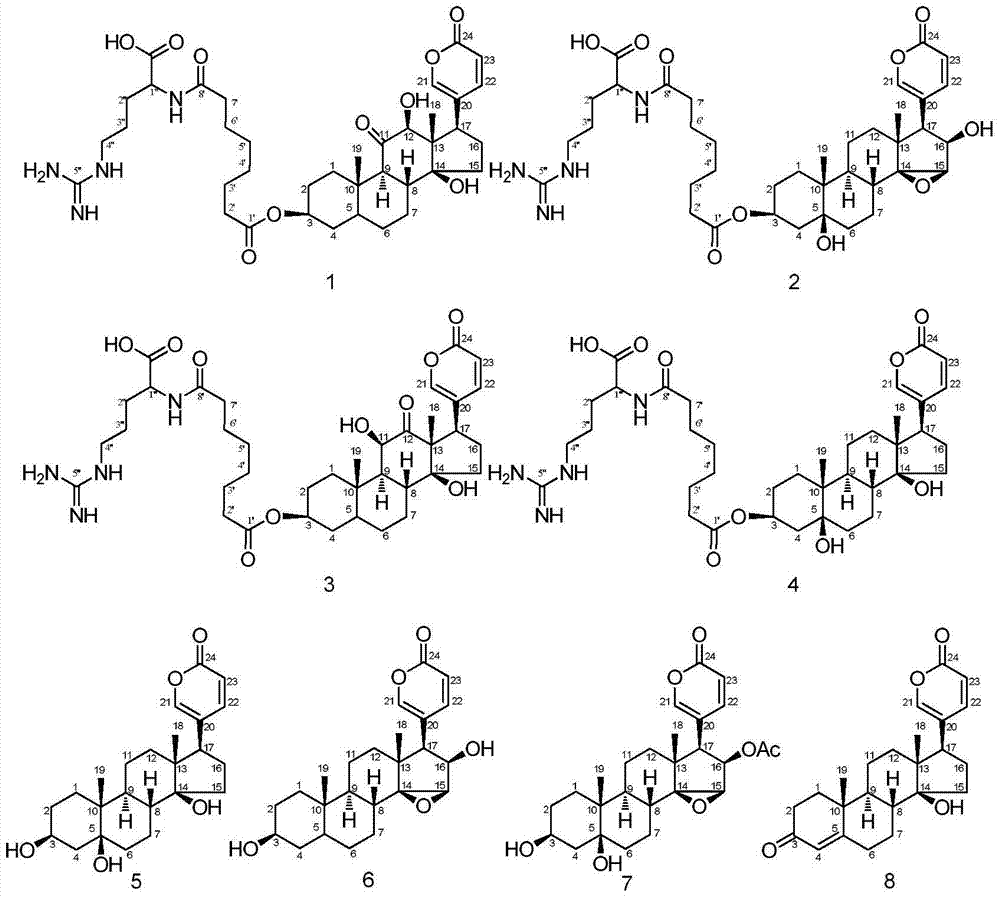
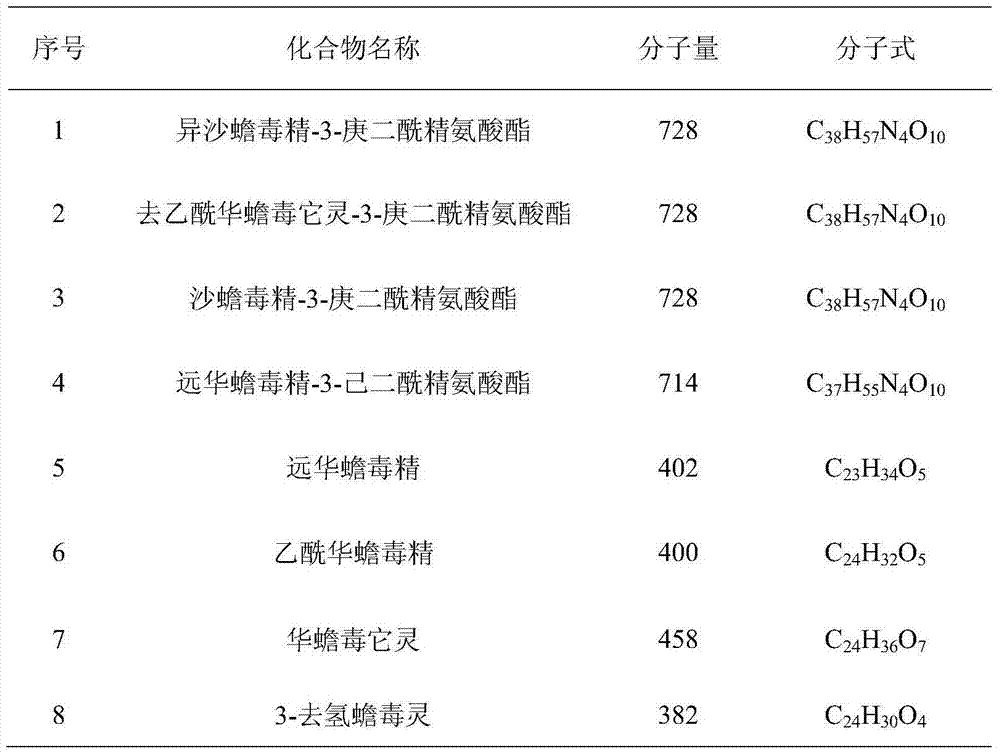
[0023] 1) The 95% (volume concentration) ethanol extract of toad skin was separated by preparative RPLC. Chromatographic conditions: chromatographic column is a carbon-octave column (C18TDE column); water (A) and methanol (B) mobile phase system; elution The gradient is 0-7min, volume concentration 25%→40%B; 7-60min, volume concentration 40%→65%B; 60-65min, volume concentration 65%→100%B; 65-75min, 100%B; flow rate It is 330mL / min; in the separation process, it is detected by an ultraviolet detector, and the detection wavelength is 300nm; it is dissolved in methanol-water with a volume concentration of 65%, and the concentration of the sample solution is 440mg / mL; the injection volume is 40mL; according to the retention of each chromatographic peak Each fraction was collected separately at different times, and a total of 22 components were collected, and each component was concentrated to dryness for later use; for the component F13 rich in bufadienolactone (the volume of metha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com