Hydrothermally stable column cage type metal organic framework material as well as preparation method and application thereof
A metal-organic framework and hydrothermally stable technology, applied in organic chemistry, separation methods, alkali metal compounds, etc., can solve the problems of inability to achieve separation and purification, low adsorption capacity, etc., and achieve good hydrothermal stability and circulation, Ultra-high adsorption capacity, high-efficiency separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
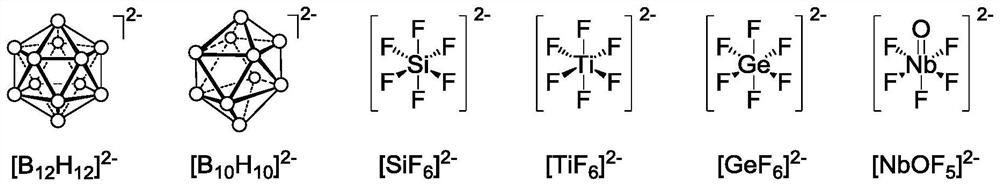
[0057] In a 50mL round bottom flask, 241.6mg (1mmol) of Cu(NO 3 ) 2 ·3H 2 O and 197.9 mg (1 mmol) of (NH 4 ) 2 TiF 6 Dissolve in 14mL of water. In another 50 mL round bottom flask, 331.05 mg (1.3 mmol) of tris(4-pyridyl)amine (L1) was dissolved in 30 mL of methanol. The methanol solution was added dropwise to the aqueous solution, and stirred at 30° C. for 48 hours to obtain a purple solid precipitate, which was filtered and washed with methanol. Soak the above solid in anhydrous methanol, replace anhydrous methanol every six hours, and replace more than 3 times in total to remove water molecules in the pores of the material, and then vacuum activate at 120°C for 10 hours to obtain a column for gas separation Cage metal-organic frameworks (CuTiF 6 ) 3 (L1) 4 .
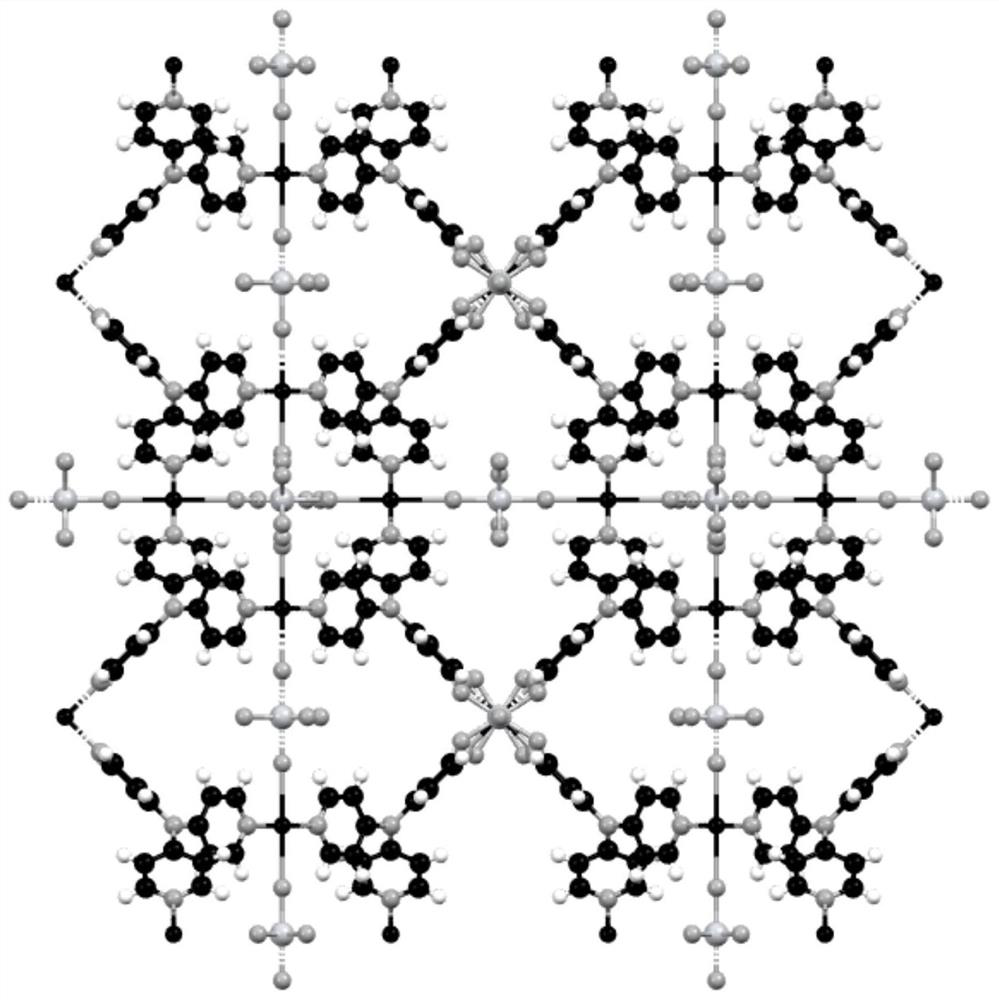
[0058] figure 2 Yes (CuTiF 6 ) 3 (L1) 4 Schematic diagram of the crystal structure of , in which copper coordinates to four different pyridine rings in the horizontal direction, and then extends infinite...
Embodiment 2
[0063] In a 50mL round bottom flask, 277.7mg (1mmol) of CuSiF 6 Dissolve in 14mL of water. In another 50 mL round bottom flask, 331.05 mg (1.3 mmol) of tris(4-pyridyl)amine (L1) was dissolved in 30 mL of methanol. The methanol solution was added dropwise to the aqueous solution, and stirred at 30° C. for 48 hours to obtain a purple-red solid precipitate, which was filtered and washed with methanol. Soak the above solid in anhydrous methanol, replace anhydrous methanol every six hours, and replace more than 3 times in total to remove water molecules in the pores of the material, and then vacuum activate at 120°C for 10 hours to obtain a column for gas separation Cage metal-organic frameworks (CuSiF 6 ) 3 (L1) 4 .
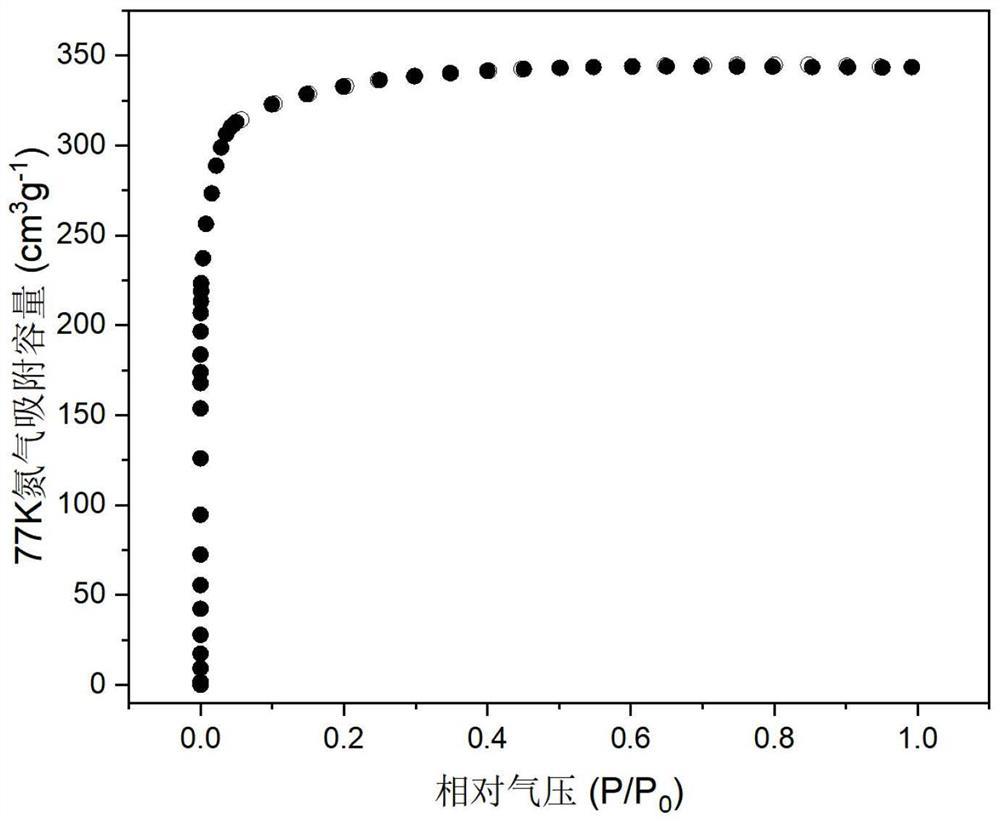
[0064] The activated material was measured at 278K, 298K, and 308K for the single-component adsorption curves of acetylene, ethylene, propyne, and propylene, and the results are as follows: Figure 5-8 shown. Then, based on the ideal adsorption solution theory...
Embodiment 3
[0067] In a 50 mL round bottom flask, 339.4 mg (1 mmol) of CuNbOF 5 Dissolve in 14mL of water. In another 50 mL round bottom flask, 331.05 mg (1.3 mmol) of tris(4-pyridyl)amine (L1) was dissolved in 30 mL of methanol. The methanol solution was added dropwise to the aqueous solution, and stirred at 30° C. for 48 hours to obtain a blue solid precipitate, which was filtered and washed with methanol. Soak the above solid in anhydrous methanol, replace anhydrous methanol every six hours, and replace more than 6 times in total to remove water molecules in the pores of the material, and then vacuum activate at 120°C for 24 hours to obtain a column for gas separation Cage metal-organic frameworks (CuNbOF 5 ) 3 (L1) 4 .
[0068] The activated materials were measured at 278K, 298K, and 308K for the single-component adsorption curves of acetylene, ethylene, propyne, and propylene, and then calculated based on the ideal adsorption solution theory (IAST) and adsorption data fitting. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com