A kind of total bufolide solid lipid nanoparticle drug delivery system for injection and its preparation method
A technology of solid lipid nanometer and total bufolide, which is applied to the digestive system, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve the problems of redness and swelling at the infusion site, poor intravenous infusion, etc., and achieve the reduction of vascular Stimulant, bioavailability-enhancing, tolerability-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
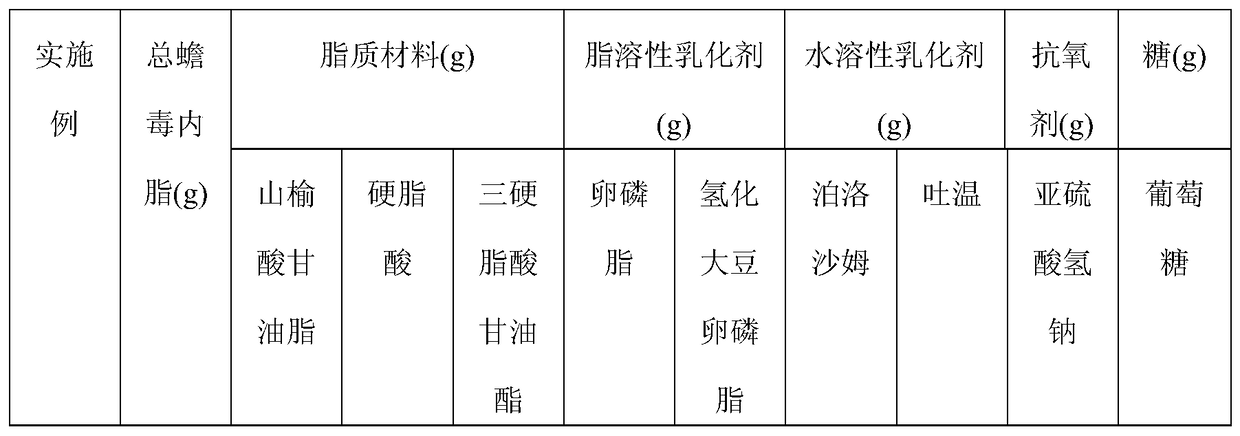
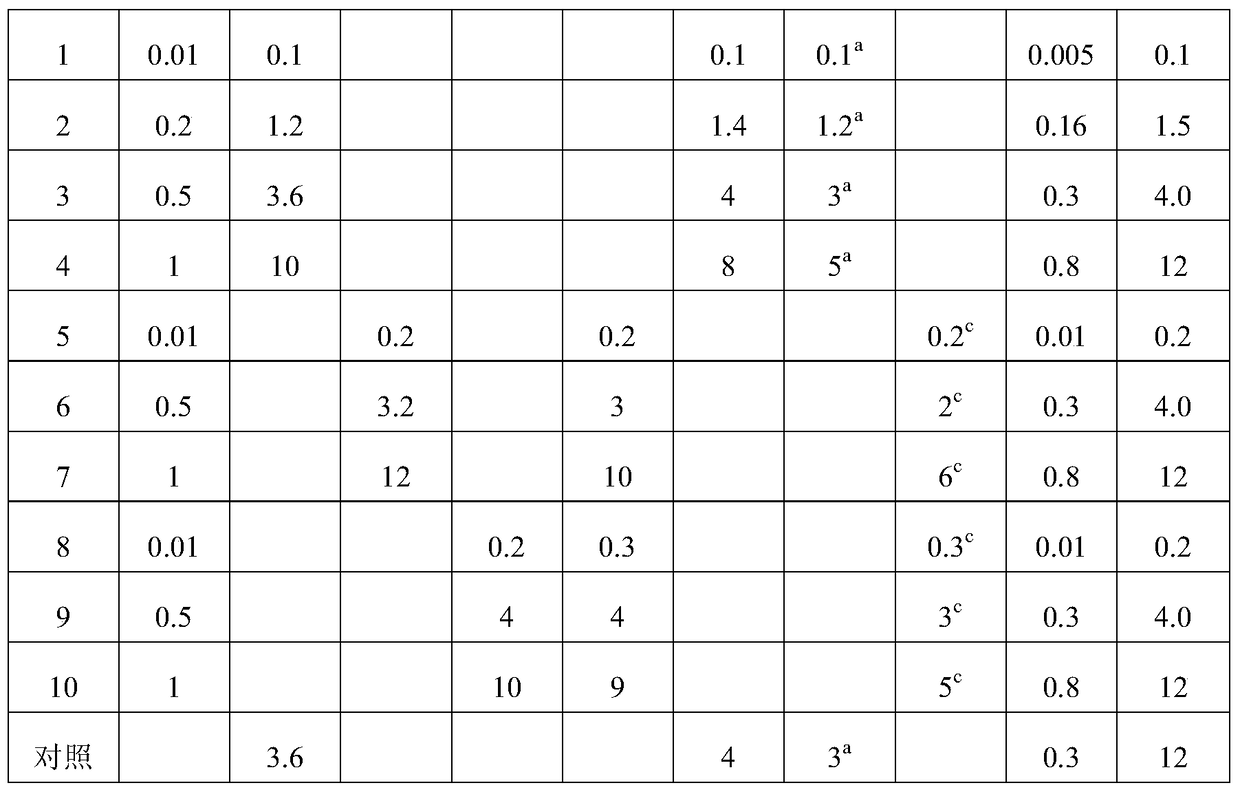
[0065] Examples 1-10 This example mainly describes the hot high-pressure emulsion homogenization method. The components specified in Table 1 were prepared according to the following steps to obtain bufogenin solid lipid nanoparticles of different examples.
[0066] (1) Heat the total bufatoxin extract, lipid material and fat-soluble emulsifier at the temperature shown in Table 2 to melt to dissolve or disperse in the lipid to prepare the melt as the oil phase;
[0067] (2) Disperse water-soluble emulsifier, antioxidant, and glucose in 100ml water for injection to form a uniform water phase;
[0068] (3) Mix and stir the oil phase obtained in (1) and the water phase obtained in (2) at the temperature and speed shown in Table 2 until uniform colostrum is formed;
[0069] (4) Transfer the colostrum prepared in (3) to a milk homogenizer, homogenize in a high-pressure milk homogenizer, and quickly cool to room temperature or below to prepare total bufogenin solid lipid nanometer grain.
[...
Embodiment 11
[0078] The solid lipid nanoparticles of total bufogenin prepared as described in the above Examples 1-10 were used for physical and chemical properties study.
[0079] Take an appropriate amount of the total bufatoxin solid lipid nanoparticle suspension, dilute it with water, and then drop it on a copper mesh covered with a carbon film, stain it with a 2.0% sodium phosphotungstate negative staining solution, and observe the nanoparticles under a transmission electron microscope Shape and take photos.
[0080] As a result, the nanoparticles were observed under the electron microscope as quasi-spherical solid particles with uniform particle size and no aggregation and adhesion.
Embodiment 12
[0082] The total bufolide solid lipid nanoparticles in Examples 1-10 were taken to determine the encapsulation efficiency and drug loading.
[0083] Sephadex glucose gel column chromatography was used to separate the total bufogenin solid lipid nanoparticles and free drugs, and determine the encapsulation efficiency. Take 1 ml of nanoparticle suspension and apply it to the column, and use distilled water as the elution medium for elution. Collect the eluate with the opalescent part, quantify the part of the eluate, add methanol to dissolve the nanoparticles; take another 1 ml of the nanoparticle suspension and add methanol to dissolve. The encapsulation efficiency and drug loading were determined by HPLC.
[0084] The particle size, encapsulation efficiency and drug loading of the total bufogenin solid lipid nanoparticles measured in Examples 11-12 are shown in the following table:
[0085] table 3
[0086] Example
[0087] The above examples are described with 100ml total bufoge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com